题目内容
I.(6分)下图表示一个食物网,请据图回答:

(1)若图中昆虫是以绿色植物的籽粒为食,野兔是以绿色植物的茎叶为食,则昆虫与野兔之间没有竞争关系,理由是 。[来源:学_科_网]
(2)一个完整生态系统的结构除图中所示的以外,还应包括 。
(3)若对图中生产者的种群密度进行调查,常用的方法是 。
II.(16分)为探究环境因素对光合作用强度的影响,有人设计并进行了以下实验。实验材料和用具:黑藻、100mL量筒、50w、100w、200w和400w的台灯、冷开水等。

实验步骤:
①准备4套如图所示装置,编号为1—4。在瓶中各加入约500mL 0.01g/mL NaHCO3溶液,再用冷开水充满。
②取4等份长势相同的黑藻分别放入l—4号装置。
③将4套装置放入暗室中,然后分别用50w、100w、200w和400w的台灯等距离地照射1—4号装置,观察气泡的产生情况。
④30min后停止光照,测量光合作用释放的氧气体积(mL)
以上实验重复三次,实验结果见上表。分析并回答有关问题:
(1)该实验中的自变量是 。因变量是 。
(2)根据装置图,上表中的实验数据是如何读出来的 。
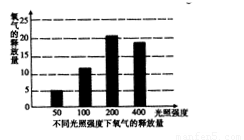
(3)根据上表中的实验数据,在右边坐标图中绘制出柱状图:

(4)上表中测得的氧气是否是光合作用实际产生的氧气量,为什么?
(5)对表中数据进行分析并得出结论: 。
(6)若想确定黑藻生长的最适光照强度,请在本实验的基础是写出进一步探究的实验思路: 。
Ⅰ.(6分)(1)它们所需的食物资源不同 (2)非生物的物质和能量,分解者。 (3)样方法
Ⅱ.(16分)(1)光照强度 O2释放量
(2)读量筒中收集到的水的体积
(3)见右图
(4)不是(2分) 黑藻自身的呼吸作用会消耗氧气,水中还会溶解部分氧气(2分)
(5)在一定范围内随着光照强度的增强,光合作用的强度也增强
(6)缩小光照梯度,在100W—400W范围内设置更多实验组
【解析】


 范围内,随着温度升高,呼吸作用 ;温度主要通过影响 的活性而影响呼吸作用。
范围内,随着温度升高,呼吸作用 ;温度主要通过影响 的活性而影响呼吸作用。 的部分代谢过程,请据图回答:
的部分代谢过程,请据图回答:
 则图中 (用字母回答)过程及其合成产物运输到块茎、块根都将受影响。
则图中 (用字母回答)过程及其合成产物运输到块茎、块根都将受影响。