��Ŀ����
5��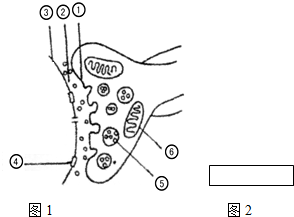 �������Ϣ����
�������Ϣ����ͼ1Ϊ��Ԫ֮�����Ϣ���ݵĽṹģʽͼ����ش�
��1��ͼ�Т١��ڡ�������ɵĽṹ������ͻ����
��2���ṹ����ṹ���ںϣ����е��������µķ�ʽ����ڣ�
��3����Ϣ����ϵͳ�еĴ��������ַ�ʽ����һ����Ԫ������������ʽ���ݣ�����Ԫ֮���ͨ������Ϊ���ʵĻ�ѧ���ʴ��ݣ����۲�λ�˷ܣ������λ�仯����ΪĤ�⸺��λ��Ĥ������λ��
��4�����ü�ͷ��ͼ2�����ڱ�ʾ��Ϣ���ݵķ��١��ڡ��ۣ�
���� ��ͼ����������ͻ��ǰĤ������ͻ����϶������ͻ����Ĥ���������壬����ͻ��С�ݣ��������壮�ݴ˷�������
��� �⣺��1��ͻ���ɢ�ͻ��ǰĤ����ͻ����϶����ͻ����Ĥ���ɣ�
��2��ͻ��С�ݢ��е����ʵ��ͷ������µķ�ʽ�����ͻ����϶�ģ�
��3���˷���һ����Ԫ������������ʽ���ݣ�����Ԫ֮���ͨ������Ϊ���ʵĻ�ѧ���ʣ��۲�λ�˷ܣ������λ�仯����Ϊ Ĥ�⸺��λ��Ĥ������λ��
��4��ͼ2��������Ϣ���ݵķ���Ϊ��ͻ��ǰĤ����ͻ����϶�ۢ�ͻ����Ĥ��
�ʴ�Ϊ��
��1��ͻ��
��2������
��3������ Ĥ�⸺��λ��Ĥ������λ
��4���١��ڡ���
���� ������ͼʾ��Ҫ����ͻ���Ľṹ������ǿ��ѧ����ͻ���ṹ�����֪ʶ���ʶ�ǡ����������ã�

��ϰ��ϵ�д�
�����Ŀ
16����ͼΪ��и������ʼ��ֲ��Ŵ�ͼ�����й۲�ֲ��ϸ����˿���ѵ���������ǣ�������
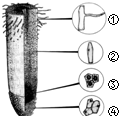

| A�� | �� | B�� | �� | C�� | �� | D�� | �� |
20����60Co����ˮ��������������Ʒ�ֵķ����У�������
| A�� | ���������� | B�� | ������� | C�� | �˹��ձ� | D�� | ��Ȼͻ�� |
10���Ŵ�������
��ͼ��ij���Ŵ�����ϵ��ͼ�����ͼ�ش𣨻�������ĸA��a��ʾ����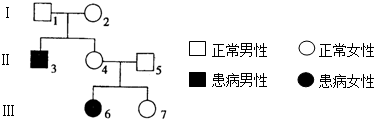
��1�����Ŵ����Ļ���λ�ڳ�����/�ԣ�Ⱦɫ���ϣ�����������/�������Ŵ���
��2��I2�Ļ�������Aa����3�Ļ�������aa��
��3����4�Ļ�������I2�Ļ�������ͬ�Ŀ�����Ϊ100%��
��4����4���5�������һ�����ӣ�����������25%��
��5���Ŵ���Σ���˵����彡������ȡ��ʩ���Խ����䷢���ʣ��±������������Ŵ������ױ���ϼ�ҽ��������ָ������Ӧ��ѡ�����B��
��ͼ��ij���Ŵ�����ϵ��ͼ�����ͼ�ش𣨻�������ĸA��a��ʾ����

��1�����Ŵ����Ļ���λ�ڳ�����/�ԣ�Ⱦɫ���ϣ�����������/�������Ŵ���
��2��I2�Ļ�������Aa����3�Ļ�������aa��
��3����4�Ļ�������I2�Ļ�������ͬ�Ŀ�����Ϊ100%��
��4����4���5�������һ�����ӣ�����������25%��
��5���Ŵ���Σ���˵����彡������ȡ��ʩ���Խ����䷢���ʣ��±������������Ŵ������ױ���ϼ�ҽ��������ָ������Ӧ��ѡ�����B��
| �Ŵ��� | �Ŵ���ʽ | �������� | ����ָ�� | |
| A | ��ά����D���Ͳ� | XȾɫ�������Ŵ� | XaXa��XAY | �������к� |
| B | ���� | ��Ⱦɫ�������Ŵ��� | Aa��Aa | ������Ů�� |
17����ϸ����Ѫ��ָ��ϸ�����Ѻ�Ѫ�쵰������������ij������Ա���˵ĺ�ϸ���ֱ����ڼ��ֵ�����Һ������ˮ���⣩�У��ⶨ��ϸ����Ѫ�����ʱ�䣬�õ���ͼ��ʾ����������йط�������ȷ���ǣ�������
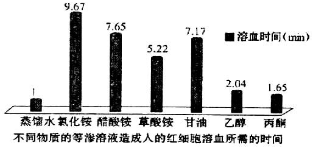

| A�� | ��ϸ��������ˮ����Ѫʱ���������Ϊ��ϸ��������ˮ��Ũ�Ȳ���� | |
| B�� | ��ͬ��Һ�к�ϸ����Ѫʱ�䳤�̿���������ͨ����ϸ��Ĥ���ٶ��й� | |
| C�� | �Ҵ������͡���ͪ�����ϸ�����ٶȴ�С��ϵΪ���ͣ��Ҵ�����ͪ | |
| D�� | �Ȼ�李�����李�������������ӵ�ͨ����������Է����������ܳ������ |
14��ij�ִ���ͬ��ֲ��Ļ�ɫ�����Ե�λ����A��a��B��b�����ƣ�ҶƬ�����ɵ�λ����D��d�����ƣ����Ի���ֱ�λ�ڲ�ͬ�Ե�ͬԴȾɫ���ϣ���֪��ɫ�����ֱ����ͣ��ϻ���A_B_�����ۻ���A_bb���Ͱ���aaB_��aabb�����±�Ϊ��ֲ�ﲿ���ӽ�ʵ��Ľ����������ش��������⣺
��1�������ϱ����ӽ�����ң����ж�ҶƬ������һ��״��խҶ��������״��
��2�����ӽ���ϲ�����F1�У��ۻ���Ҷֲ��Ļ�������AAbbDd��AabbDd�� ���ӽ�������ױ��ϻ���Ҷֲ��Ļ�������AABbDd��
��3����ֻ���ǻ�ɫ���Ŵ������������F1��ȫ���ϻ�ֲ���Խ������Ӵ�ֲ��Ļ�������9�֣����зۻ�ֲ����ռ�ı���Ϊ$\frac{1}{8}$��
��4����ֲ����Ȼ״̬�¼������ɽ���������ӽ�����ֻ����ҶƬ���ȵ��Ŵ����ֽ����ֿ�Ҷ��խҶֲ���ӽ���������F1�����Խ�����F2����
������F2��ֲ�����Խ��������Ӵ���״���ּ������ǿ�Ҷ��խҶ=5��3��
������F2�������п�Ҷֲ�����ɽ��䣬�����Ӵ���״���ּ������ǿ�Ҷ��խҶ=8��1��
| ��� | �ױ� ��� | F1�ı����ͼ����� | |||||
| �ϻ���Ҷ | �ۻ���Ҷ | ����Ҷ | �ϻ�խҶ | �ۻ�խҶ | ��խҶ | ||
| �� | �ϻ���Ҷ���ϻ�խҶ | $\frac{9}{32}$ | $\frac{3}{32}$ | $\frac{4}{32}$ | $\frac{9}{32}$ | $\frac{3}{32}$ | $\frac{4}{32}$ |
| �� | �ϻ���Ҷ������Ҷ | $\frac{9}{16}$ | $\frac{3}{16}$ | 0 | $\frac{1}{16}$ | $\frac{1}{16}$ | 0 |
| �� | �ۻ���Ҷ���ۻ�խҶ | 0 | $\frac{3}{8}$ | $\frac{1}{8}$ | 0 | $\frac{3}{8}$ | $\frac{1}{8}$ |
��2�����ӽ���ϲ�����F1�У��ۻ���Ҷֲ��Ļ�������AAbbDd��AabbDd�� ���ӽ�������ױ��ϻ���Ҷֲ��Ļ�������AABbDd��
��3����ֻ���ǻ�ɫ���Ŵ������������F1��ȫ���ϻ�ֲ���Խ������Ӵ�ֲ��Ļ�������9�֣����зۻ�ֲ����ռ�ı���Ϊ$\frac{1}{8}$��
��4����ֲ����Ȼ״̬�¼������ɽ���������ӽ�����ֻ����ҶƬ���ȵ��Ŵ����ֽ����ֿ�Ҷ��խҶֲ���ӽ���������F1�����Խ�����F2����
������F2��ֲ�����Խ��������Ӵ���״���ּ������ǿ�Ҷ��խҶ=5��3��
������F2�������п�Ҷֲ�����ɽ��䣬�����Ӵ���״���ּ������ǿ�Ҷ��խҶ=8��1��
15�������深ֳ���ڣ�ʹ���ض��������ռ������Խ�ij������IJ������Գɳ���ɱ�������벻ʹ�������ռ���ȣ����ǣ�������
| A�� | �ı��Ӵ����Ա���� | B�� | �ı��Ӵ�����Ⱥ�ܶ� | ||
| C�� | ����ı������ | D�� | �ı��Ӵ���Ⱥ�Ŀռ����� |