��Ŀ����
�������������������ʵ�飬�ش�������⡣
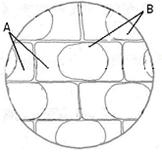
��1���ʱڷ���ʵ�飺���������ƬҶ�ڱ�Ƥ����ʱװƬ���ӸDz�Ƭһ�������֬����Һ��ֲ��ϸ�������յĺ�ɫȾ�ϣ�������һ������ˮֽ�������ظ����Σ�ʹ���Ͻ�������֬����Һ�С������۲�����ͼ��
A��B������ɫ�ֱ��ǣߣߣߣߡ��ߣߣߣߡ���������Ƭ����80���´���һ��ʱ���۲죬����B����ɫ�����仯��ԭ������ǣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߡ�
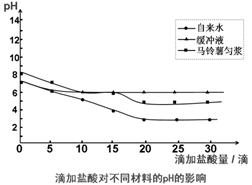
��2���о�������ά��pH�ȶ��Ļ��ƣ������������ˮ�����建���������Ƚ��У����μ�0.1mol/L��HCl��NaOH��Һ��pH�仯���±���
| pH ʵ�� ���� | ����0.1mol/L HCl���� | ����0.1mol/L NaOH���� | ||||||||||||
| 0 | 5 | 10 | 15 | 20 | 25 | 30 | 0 | 5 | 10 | 15 | 20 | 25 | 30 | |
| ����ˮ | 7 | 6 | 5 | 4 | 3 | 3 | 3 | 7 | 7 | 8 | 9 | 10 | 11 | 12 |
| ����Һ | 7 | 6 | 6 | 6 | 6 | 6 | 6 | 7 | 7 | 7 | 7 | 7 | 7 | 7 |
| �������Ƚ� | 8 | 7 | 6 | 6 | 5 | 5 | 5 | 8 | 9 | 9 | 9 | 9 | 9 | 9 |
��1����ɫ����ɫ�����ɫ�����֣���������ϸ��ʧ���ˣ���ϸ��Ĥʧȥѡ�����ԣ�����֬�����B����
��2���������Ƚ����л������ã���������Ͼ����뻺��Һ���Ƶ�ά��PH�ȶ���������
��3��Ưϴ��Ⱦɫ����Ƭ����������״̬��װƬ��״��������̶Ȼ�Ⱦɫ�������ϸ�����ڸ�ʱ�ڵ�����/��Ұ�IJ�ͬ������������㼴�ɣ�
��4���������Ƚ���������֤���۵Ĵ��ڣ���֤��������ø�Ĵ����ԣ�������ĸ��������������и���������֤�����յ�Ⱦɫ����Ŀ�ӱ����ڱ�Ƥ���ڹ۲�DNA��RNA�ķֲ����������һ�㼴�ɣ���������ʵ��Ҳ�ɸ��֣�

�������������������ʵ�飬�ش�������⡣
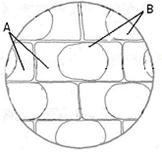
��1���ʱڷ���ʵ�飺���������ƬҶ�ڱ�Ƥ����ʱװƬ���ӸDz�Ƭһ�������֬����Һ��ֲ��ϸ�������յĺ�ɫȾ�ϣ�������һ������ˮֽ�������ظ����Σ�ʹ���Ͻ�������֬����Һ�С������۲�����ͼ��

A��B������ɫ�ֱ��ǣߣߣߣߡ��ߣߣߣߡ���������Ƭ����80���´���һ��ʱ���۲죬����B����ɫ�����仯��ԭ������ǣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߡ�
��2���о�������ά��pH�ȶ��Ļ��ƣ������������ˮ�����建���������Ƚ��У����μ�0.1mol/L��HCl��NaOH��Һ��pH�仯���±���
|
pH ʵ�� ���� |
����0.1mol/L HCl���� |
����0.1mol/L NaOH���� |
||||||||||||
|
0 |
5 |
10 |
15 |
20 |
25 |
30 |
0 |
5 |
10 |
15 |
20 |
25 |
30 |
|
|
����ˮ |
7 |
6 |
5 |
4 |
3 |
3 |
3 |
7 |
7 |
8 |
9 |
10 |
11 |
12 |
|
����Һ |
7 |
6 |
6 |
6 |
6 |
6 |
6 |
7 |
7 |
7 |
7 |
7 |
7 |
7 |
|
�������Ƚ� |
8 |
7 |
6 |
6 |
5 |
5 |
5 |
8 |
9 |
9 |
9 |
9 |
9 |
9 |
����������ϵ�У���ͼ��ӳ�μ�HCl�����ֲ���pH��Ӱ�졣�ݴ˵ó��Ľ����ǣ��ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߡ�

��3���۲���˿���ѣ�����и���������ʱװƬ�������ǣ�������ߣߣߣߣߣߣߣߣߣߣߣߡ���λͬѧͬʱ����ʵ�飬������ͬ��������ͳ��50��ϸ����������ͬ����ʱ�ڵ�ϸ�����������ֽ���в��죮�������ֲ���ľ���ԭ������У��٣ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣ��ڣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߡ�
��4��������������������ϣ������������һ������ѧʵ�飿�ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣ�