��Ŀ����
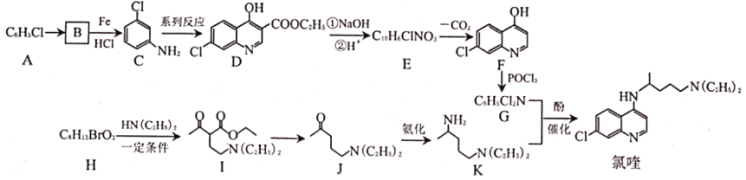
����Ŀ������ɽԺʿ˵��������������¹ڷ�����Ч���������������������ᷴӦ�Ƶá��ϳ���୵�·������ͼ��ʾ����ش��������⣺
��֪���� ����
���� ��
��![]() �������ƣ�
�������ƣ�
��![]()
![]() ��R��
��R��![]() ��ʾ������R�@��ʾ�������⣬
��ʾ������R�@��ʾ�������⣬![]() ��ʾ±ԭ�ӣ���
��ʾ±ԭ�ӣ���
��1��A��������________��D�еĺ���������������________��
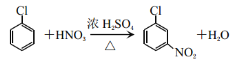
��2��![]() ��୵ķ�Ӧ������________��
��୵ķ�Ӧ������________��
��3��![]() �Ľṹ��ʽΪ________��
�Ľṹ��ʽΪ________��
��4��д����A����B�Ļ�ѧ��Ӧ����ʽ________��
��5����D����E�ĵڢٲ���Ӧ�Ļ�ѧ����ʽΪ________��
��6��![]() ��
��![]() ��ͬϵ���Է���������
��ͬϵ���Է���������![]() ��14��
��14��![]() ��ͬ���칹���У��������������ֹ����ţ�����������Ϊ��ԭ�ӺͰ����Ľṹ��________�֣����������칹����
��ͬ���칹���У��������������ֹ����ţ�����������Ϊ��ԭ�ӺͰ����Ľṹ��________�֣����������칹����
��7����������·�ߣ�������Ա��ͻ���ͪΪԭ�Ϻϳ�![]() ��·�ߣ�________��
��·�ߣ�________��
���𰸡��ȱ� �ǻ��� ���� ȡ����Ӧ ![]()
 +2NaOH
+2NaOH![]()
 +CH3CH2OH+H2O 20
+CH3CH2OH+H2O 20 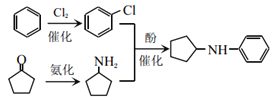
��������
��������ͼ��C�Ľṹ��ʽ��B��Cת���������������֪�ٿ�ʵ��B��C��ת�����ٽ��A�ķ���ʽ�����Եó�A��B�Ľṹ��ʽ�ֱ�Ϊ��![]() ��
�� ������D��E��ת��������E�ķ���ʽ��������֪EΪ��
������D��E��ת��������E�ķ���ʽ��������֪EΪ��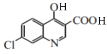 ��E�������ȷ�Ӧ���ɵõ�F������F����୵Ľṹ��ʽ�����Ƴ�G�Ľṹ��ʽΪ��
��E�������ȷ�Ӧ���ɵõ�F������F����୵Ľṹ��ʽ�����Ƴ�G�Ľṹ��ʽΪ��![]() ������H�ķ���ʽ��I�Ľṹ��ʽ��H��I��ת�������������֪�ۿ�֪H�Ľṹ��ʽΪ��
������H�ķ���ʽ��I�Ľṹ��ʽ��H��I��ת�������������֪�ۿ�֪H�Ľṹ��ʽΪ��![]() ���ݴ˷�������
���ݴ˷�������
��1��A�Ľṹ��ʽ![]() ��������Ϊ�ȱ���D�Ľṹ��ʽΪ
��������Ϊ�ȱ���D�Ľṹ��ʽΪ![]() �����京�������������Ƿ��ǻ����������ʴ�Ϊ���ȱ������ǻ���������
�����京�������������Ƿ��ǻ����������ʴ�Ϊ���ȱ������ǻ���������
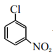
��2��![]() ��୵ķ�ӦΪ��
��୵ķ�ӦΪ��![]() +
+ 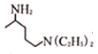 ��
��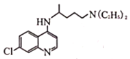 +HCl���ʷ�Ӧ��������ȡ����Ӧ���ʴ�Ϊ��ȡ����Ӧ��
+HCl���ʷ�Ӧ��������ȡ����Ӧ���ʴ�Ϊ��ȡ����Ӧ��
��3�����ݷ�����֪��![]() �Ľṹ��ʽΪ��
�Ľṹ��ʽΪ��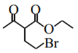 ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
��4�����ݷ�����A��B�Ľṹ��ʽ�ֱ�Ϊ��![]() ��
�� ������A����B�Ļ�ѧ��Ӧ����ʽΪ��
������A����B�Ļ�ѧ��Ӧ����ʽΪ��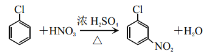 ���ʴ�Ϊ��
���ʴ�Ϊ��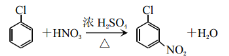 ��
��
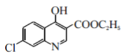
��5�����������ͼ��֪��D�Ľṹ��ʽΪ![]() ������E�ĵڢٲ���Ӧ�����ڼ���������ˮ�ⷴӦ�����仯ѧ����ʽΪ
������E�ĵڢٲ���Ӧ�����ڼ���������ˮ�ⷴӦ�����仯ѧ����ʽΪ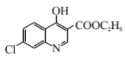 +2NaOH
+2NaOH![]()
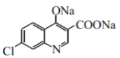 +CH3CH2OH+H2O���ʴ�Ϊ��
+CH3CH2OH+H2O���ʴ�Ϊ�� +2NaOH
+2NaOH![]()
 +CH3CH2OH+H2O��
+CH3CH2OH+H2O��
��6��![]() Ϊ
Ϊ![]() ��ͬϵ� ��Է���������
��ͬϵ� ��Է���������![]() �� 14�� ��
�� 14�� �� ![]() ��B��һ��
��B��һ��![]() ��
�� ![]() ��ͬ���칹���У� �������������������ţ����������ֱ�Ϊ��������ԭ�ӣ� ��һ��ȡ��������Ϊ
��ͬ���칹���У� �������������������ţ����������ֱ�Ϊ��������ԭ�ӣ� ��һ��ȡ��������Ϊ![]() ��
��![]() �� �����ϰ�������ԭ�ӵ�λ�����ڡ� �䡢�� 3 �֣� ��һ��ȡ������λ�÷ֱ��� 4�� 4�� 2 �֣� ������������ͬ���칹���� 20 �֣��ʴ�Ϊ��20
�� �����ϰ�������ԭ�ӵ�λ�����ڡ� �䡢�� 3 �֣� ��һ��ȡ������λ�÷ֱ��� 4�� 4�� 2 �֣� ������������ͬ���칹���� 20 �֣��ʴ�Ϊ��20
��7�����ǿ��Բ������Ʒ���ƺϳ�·�ߣ�Ŀ�����Ϊ![]() �����������
�����������![]() �����֪�ۿ�֪����
�����֪�ۿ�֪����![]() ��
��![]() ����ȡ����Ӧ�õ�
����ȡ����Ӧ�õ�![]() ������Cl2����ȡ����Ӧ���Ƶ��ȱ�������ͪ
������Cl2����ȡ����Ӧ���Ƶ��ȱ�������ͪ![]() ��
��![]() ����Դ��������ͼ��J��K�IJ������ҵ���Ϣ��������ȷ���ϳɵ�����ͼΪ��
����Դ��������ͼ��J��K�IJ������ҵ���Ϣ��������ȷ���ϳɵ�����ͼΪ�� ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��