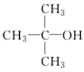
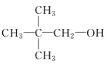
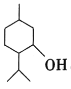
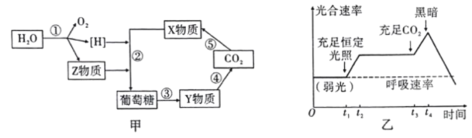
��Ŀ����
����Ŀ��A(C3H6)�ǻ����л�����ԭ�ϡ���A�Ʊ��ۺ���C��![]() �ĺϳ�·��(���ַ�Ӧ������ȥ)��ͼ��ʾ��
�ĺϳ�·��(���ַ�Ӧ������ȥ)��ͼ��ʾ��

��֪��![]() +
+![]()
![]()
![]() ��R-C��N
��R-C��N![]() R-COOH
R-COOH
�ش��������⣺
(1)A��������_______��B�к��������ŵ�������__________��
(2)C�Ľṹ��ʽΪ_______________��D��E�ķ�Ӧ����Ϊ________��
(3)E��F�Ļ�ѧ����ʽΪ_________��
(4)![]() �������۷�Ӧ�����л���Ľṹ��ʽΪ_________��
�������۷�Ӧ�����л���Ľṹ��ʽΪ_________��
(5)B��ͬ���칹���У���B������ͬ�Ĺ��������ܷ���������Ӧ�Ĺ���________�֣����к˴Ź�������Ϊ3��壬�ҷ����֮��Ϊ6��1��1����__________(д�ṹ��ʽ)��
���𰸡���ϩ ����  ȡ����Ӧ��ˮ�ⷴӦ
ȡ����Ӧ��ˮ�ⷴӦ  +
+
![]()
![]()
![]() 8
8 ![]()
��������
��B��Ӧ�õ��ĸ߾���Ľṹ����֪B�����Ӿ۷�Ӧ�õ��߾���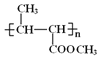 ����BΪCH3CH=CHCOOCH3�����A�ķ���ʽ����֪AΪCH2=CHCH3��
����BΪCH3CH=CHCOOCH3�����A�ķ���ʽ����֪AΪCH2=CHCH3��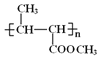 ����ˮ�ⷴӦ���ữ�õ��ۺ���C����CΪ
����ˮ�ⷴӦ���ữ�õ��ۺ���C����CΪ ��A�������ڸ����·�Ӧ����D��D����ˮ�ⷴӦ����E��E�ܷ�����Ϣ�еļӳɷ�Ӧ�����E�ķ���ʽ��G�IJ���(
��A�������ڸ����·�Ӧ����D��D����ˮ�ⷴӦ����E��E�ܷ�����Ϣ�еļӳɷ�Ӧ�����E�ķ���ʽ��G�IJ���(![]() )�Ľṹ�������֪��Ϣ��֪DΪCH2=CHCH2Cl��EΪCH2=CHCH2OH��FΪ
)�Ľṹ�������֪��Ϣ��֪DΪCH2=CHCH2Cl��EΪCH2=CHCH2OH��FΪ![]() ��GΪ
��GΪ![]() ���ݴ˷������
���ݴ˷������
(1)AΪCH2=CHCH3��A������Ϊ��ϩ��BΪCH3CH=CHCOOCH3��B�к������������������ʴ�Ϊ����ϩ��������
(2)C�Ľṹ��ʽΪ�� ��D��E��±������ˮ�ⷴӦ��Ҳ����ȡ����Ӧ���ʴ�Ϊ��
��D��E��±������ˮ�ⷴӦ��Ҳ����ȡ����Ӧ���ʴ�Ϊ�� ��ȡ����Ӧ(��ˮ�ⷴӦ)��
��ȡ����Ӧ(��ˮ�ⷴӦ)��
(3)E��F�Ļ�ѧ����ʽΪ�� +
+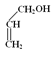
![]()
![]() ���ʴ�Ϊ��
���ʴ�Ϊ�� +
+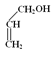
![]()
![]() ��
��
(4)![]() �������۷�Ӧ�����л���Ľṹ��ʽΪ��
�������۷�Ӧ�����л���Ľṹ��ʽΪ��![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
(5)B�ṹ��ʽΪCH3CH=CHCOOCH3��B��ͬ���칹����B������ͬ�Ĺ��������ܷ���������Ӧ��˵������̼̼˫����������ȩ����Ϊ������������������ͬ���칹����HCOOCH=CHCH2CH3��HCOOCH2CH=CHCH3��HCOOCH2CH2CH=CH2��HCOOC(CH3)=CHCH3��HCOOCH=C(CH3)2��HCOOCH(CH3)CH=CH2��HCOOCH2C(CH3)=CH2��HCOOC(CH2CH3)=CH2�����Է�����������8�֣����к˴Ź�������Ϊ3��壬�ҷ����֮��Ϊ6��1��1����![]() ���ʴ�Ϊ��8��
���ʴ�Ϊ��8��![]() ��
��