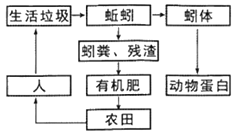
��Ŀ����
����Ŀ���������ϴ���ʷ�ƾá����߷�ζ��������������������ͼ��
��ش��������⣺
(1)���ǻ�������ø�Ƽ���Ҫ���Ʒ�Ӧ�¶ȣ�������Ϊø______________________��
(2)�ھƾ����ͽΣ������ӽ�ĸ�����ڲ��������У�������ͨ�������ܱա�ͨ�������________���������������ܱ�ʱ��ø���ľƾ����
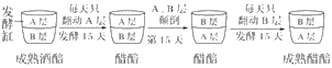
(3)�ڴ��ᷢ�ͽΣ������ϴײ��ö��صķֲ���巢�ͷ�������30�졣�������£�
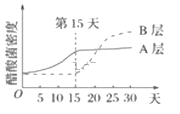
�ٷ������У�����ȡ���ⶨ������ܶȱ仯��������ͼ����ͼ��������ߵ�ǰ��ȣ�B�������ڵߵ����ܶȱ仯���ص���____________________________���ɴ��Ʋ⣬Ӱ�������ܶȱ仯����Ҫ����������__________________��
�����Ậ�����Ƕ����ϴ�ζ���ص���Ҫ���������У�������____________��Ĵ����������������ֳ���������ᡣ��ĸ����������������������ԭ���������____________��
�۳����������������������Լ��٣���Ҫԭ���Ƿ��ͺ���Ӫ���������ĵȻ������صı仯���Ӿ��˲�ͬ�����������__________����̭�˲�����������ࡣ
���𰸡��������¶������´�������ǿ ��ĸ�� �ȿ��������������ȶ� ������Ӫ�����ʡ�pH �ߵ�ǰ��B��͵ߵ����A(����������) ������������ �ּ侺��(����)
��������
1��������������������ǽ�ĸ�������³´�л����Ϊ�������������͡�����������ԭ����
��1�������������£���Ӧʽ���£�C6H12O6+6H2O+6O2![]() 6CO2+12H2O+������
6CO2+12H2O+������
��2�������������£���Ӧʽ���£�C6H12O6![]() 2CO2+2C2H5OH+������
2CO2+2C2H5OH+������
2��������������������Ǵ���������³´�л���������������͡�����������ԭ����
����������Դ������ʱ�������������֭�еĹ��Ƿֽ�ɴ��ᡣ
��ȱ����Դʱ����������Ҵ���Ϊ��ȩ���ٽ���ȩ��Ϊ���ᡣ
��1����������ͼ��֪���ǻ��ξ��ǵ����ڵ���ø�Լ���ѿ��ø��������ˮ����������ǣ������������¶�������ø�Ĵ�������ǿ��������ǻ�������ø�Ƽ���Ҫ���Ʒ�Ӧ�¶ȡ�
��2����ĸ�����ڼ���������������������������������ܹ�������ֳ�������������������������������ƾ�������ھƾ����ͽΣ�������ͨ�������ܱա�ͨ����Ŀ������߽�ĸ�����������������ܱ�ʱ��ø���ľƾ����
��3���ٴ��������������ϸ����ֻ�������������ʱ���ܹ�������ֳ���ܲ������ᡣ��ͼ��������ߵ�ǰ��ȣ�B�����˾��ܶ��ȿ��������������ȶ���Ӱ�������ܶȱ仯����Ҫ����������������Ӫ�����ʡ�pH��
�����Ậ�����Ƕ����ϴ�ζ���ص���Ҫ�������ᷢ������������е�������������˷������У������еߵ�ǰ��B��͵ߵ����A�����������£�������������������ֳ���������ᡣ����˾������������ԭ���������ĸ�����������������ڽṹ�ϵ���Ҫ�����ǣ���������������Ĥ��Χ��ϸ���ˣ�����ĸ���С�
�۳����������������������Լ��٣���Ҫԭ���Ƿ��ͺ���Ӫ���������ĵȻ������صı仯���Ӿ��˲�ͬ������������ּ侺��������������̭�˲�����������ࡣ

 �Ķ��쳵ϵ�д�
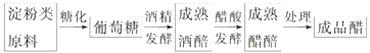
�Ķ��쳵ϵ�д�����Ŀ����Ƴ�����������øȥ���ƾ�����Ʒ�е����أ��Ը��ƾƾ�����Ʒ��Ʒ�ʡ���ش��������⣺
��1����������ɸѡ������ø��������������������Ҫ�ɷ��������ǡ����ء���֬�ȣ��ӹ����Ϸ�����������������________�����������������ĵ�Դ����_________��
��2��ɸѡ�ʹ���������ø���������õ����ֽ��ַ�����___________��_____________������Ũ���ݶ�Ϊ10-1��ϵ��ϡ��Һʱ��������Һ�ܽ�lmL��Һ����ʢ��____mL����ˮ���Թ��С�
��3����ͬһŨ�ȵ�������ø������ϡ��Һ���ֱ���Ѫ������������ϡ��Ϳ��ƽ�巨��������������ʵ�����������ǰ�ߵ�����_______�����ڣ����ڣ�С�ڣ����ߣ���ԭ����________________________��
��4�����ú������ƹ̶���������ø��ϸ��ʱ��Ӧ������������Һ��ϸ����ϵ�����Һ�С��±��Dz�ͬŨ�Ⱥ��������γɵ�������״�����ӱ��п������������Ƶ�Ũ��Ӧѡ��__________g��dL�������ù̶���ø�����������ŵ���___________________��
������������Ũ�ȣ�g/dL�� | ����ǿ�� | ���������� | �����Ѷ� | ������״ |
1.0 | �� | û�� | ���� | ��Բ�� |
2.0 | �� | ���� | �� | Բ���� |
3.0 | ǿ | �϶� | ���� | ����β |
4.0 | ǿ | ���� | �� | �ѳ����� |