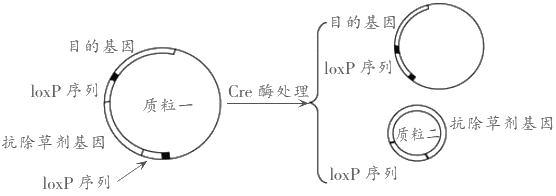
��Ŀ����
����Ŀ�������������ϲ��ش����⣺
����һ��

��ͼ��R��R����R������R��������������ͬ����ͬ��ԭ���Ż�̼����A��B��C��D�ֱ��ʾ��ͬ�Ļ�ѧ����
���϶�������һ���ģ���ѧʽΪC55H70O19N10����֪��������ˮ���õ��������ְ����
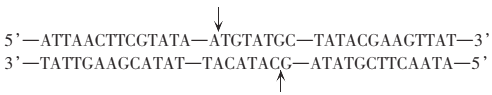
(1)��������һ���û���������_______��������ʧȥ_______����ˮ���γɣ�ˮ�����е���������____________����������_______________��
(2)��������һ���û����ﹲ���ļ�________�����ڸö���ˮ��ʱ�����ѵĻ�ѧ����___________����ͼ����ĸ��ʾ����
(3)�������϶���������Ȱ����R���ֱ���_____________��______________��
(4)�������϶��иö��Ļ�ѧʽ���㣬������ȫˮ���ɵõ�������__________�������б�����__________����
���𰸡�4 3 �Ȼ� �������Ȼ� 3 C -CH3 - CH2-CH2-COOH 10 2
��������
��������һ�е���ͼ������һ��ͼ��R��R����R������R��������������ͬ����ͬ��ԭ���Ż�̼������R�������˻���������5���������γɵ����ģ����Ӹ����������Ļ�ѧ�����ļ�����4�����ļ��Ľṹ�ɼ�дΪ-CO-NH-��
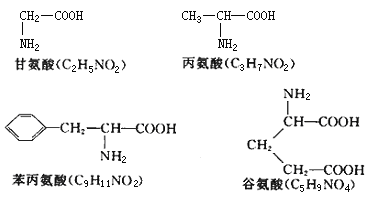
�������϶��е���ͼ�����϶��е����ְ������ǻ�ѧʽΪC54H73O22N11�Ķ��ij���ˮ���õ��ģ������ְ����ᶼֻ����һ��Nԭ�ӣ�R���о�������������ֻ�йȰ��Ậ��4����ԭ�ӣ�R���к���1���Ȼ������������ְ������������2����ԭ����
��1��������һ����ͼ��֪���û�������ļ�����3�����������4����������ȥ3����ˮ�γɵ����Ļ������������ˮ����ʱ��һ��������İ�������һ����������Ȼ���Ӧ��ȥ1����ˮ�����ˮ�е�O�����Ȼ���H���������Ȼ���
��2��������һ����ͼһ��֪���û�������ļ�����3�����ڸö���ˮ��ʱ�����ѵĻ�ѧ�����ļ�����ͼ�е�C��
��3�������϶�����ͼ��֪���������R����-CH3���Ȱ����R����-CH2-CH2-COOH��
��4�������϶�����ͼ��֪����ѧʽΪC55H70O19N10����ˮ���õ��İ����ᶼֻ����һ��N�������ȫˮ��õ��İ�������Ŀ��10��������ֻ�йȰ��Ậ��4��O����˹Ȱ�����Ŀ��[��19+9��-20]��2=4������û����ﺬ��X���ʰ��ᡢY�������ᡢZ���������ᣬ����Ԫ���غ�ɵù�ϵʽ��X+Y+Z=6��2X+3Y+9Z=35��5X+7Y+11Z+36-18=70�����X=1��Y=2��Z=3��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�