��Ŀ����
����Ŀ�����м�ͼ��DNA������a��d������������ͼ��ijһƬ�ηŴ������ͼ��ʾ�������ѧ֪ʶ�ش��������⣺
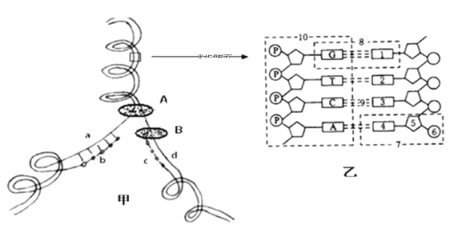
��1���Ӽ�ͼ�ɿ���DNA���Ʒ�ʽ��____________��
��2������N������Ե�һ��DNA����Ƭ���У�����m�����������������ᣬ���Ƭ����ɵ�n�θ�����Ҫ______________������İ�������������ᡣ
��3����ͼ�У�A��B����DNA���Ӹ��ƹ���������Ҫ��ø������B�ܽ��������������������ӳ����������������Ӷ��γ���������A��_______________ ø��B��_____________ø����ͼ��7��������________________��
��4��DNA���Ӿ�ȷ���Ƶ�ԭ��_______________________________Ϊ�����ṩ�˾�ȷ��ģ�壬_________________________________��֤�˸����ܹ�ȷ�Ľ��С�
���𰸡��뱣������ 2n-1����N-m�� ����ø DNA�ۺ�ø ����������������� DNA���ص�˫�����ṹ ����������ԭ��
��������
������ͼ��֪����ͼ��ʾDNA���ӵĸ��ƹ��̣�����A�ǽ���ø��B��DNA�ۺ�ø��a��d��DNA���Ƶ�ģ������b��c���ºϳɵ���������ͼ��֪DNA���Ӹ����DZ߽����߸��Ƶģ��Ҿ��а뱣�����Ƶ��ص㣻ͼ��DNA���ӽṹ��ƽ��ͼ������1�Ǽ��C��2�Ǽ��A��3�Ǽ��G��4�Ǽ��T��5���������ǣ�6�����ᣬ7����������������Ǻ����ᣬ8�Ǽ���ԣ�9�������10���������Ǻ���������
��1����ͼ��������ͼ��DNA�ĸ��Ʒ�ʽ�ǰ뱣�����ƣ��ӹ��̿��DZ߽����߸��Ƶġ�
��2����֪һ��DNA����Ƭ���о���N������ԣ���2N�����������������������������m������A=T=m����G=C=N-m������˸�Ƭ����ɵ�n�θ�����Ҫ����İ����������������=2n-1����N-m������
��3���������Ϸ�����֪����ͼ��A�ǽ���ø��B��DNA�ۺ�ø����ͼ��7������������������ᡣ
��4��DNA���ƹ����У�DNA���Ӷ��ص�˫�����ṹΪ�����ṩ�˾�ȷ��ģ�壬ͨ�����������Ա�֤�˸���ȷ����ؽ��С�