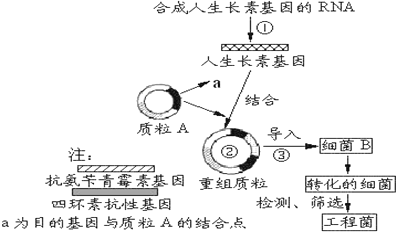
��Ŀ����
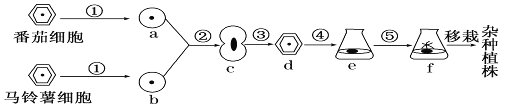
����Ŀ����ͼΪֲ����ϸ���ӽ�����ʾ��ͼ�����ͼ�ش�
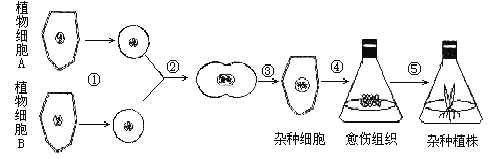
��1��ֲ����ϸ���ӽ��ĵڢٲ���ȥ��ϸ����,������л�����ԭ�����塣Ŀǰ�˲�����õķ�����ø�ⷨ��Ҳ�������º͵���������_______________�ȷֽ�ֲ���ϸ���ڡ�
��2���ڹ��̵ķ�����������˹��յ����˹��յ�ԭ�������ںϵ���������������_______�ȣ�����д�����֣���ʹԭ�����ںϣ���ѧ��������_____���Լ���Ϊ�յ����յ��ںϡ�����ϸ���ں���ֲ���������ںϵĻ���ԭ����ͬ���յ��ںϵķ������ƣ�����ϸ�����ںϻ����õ�_____��Ϊ�յ�����
��3���۱�ʾ�ںϺ��ԭ�������ٲ����µ�ϸ���ڣ���ϸ���ڵIJ�����ϸ����________(�ϸ���������ơ�)�����й�ϵ��
��4���ڢܢݹ����У�ϸ�����ѵ���Ҫ��ʽ����˿���ѣ����ַ��ѷ�ʽ����Ҫ������______________________��
��5��ֲ����ϸ���ӽ������ֹ����о��й㷺��Ӧ�ü�ֵ����ͻ�����ŵ��ǿ��� ___________��Ŀǰ������ϸ���ںϼ�������Ҫ����;��__________��
���𰸡���ά��ø������ø ���ġ����缤 ���Ҷ��� ���IJ��� �߶����� �״�ϸ����Ⱦɫ�徭�����ƺ�ȷ��ƽ�����䵽������ϸ����ȥ �˷�ԶԵ�ӽ����ϵ��ϰ� �Ʊ�����¡����
��������
������������ͼʾ������֪�����⿼��ѧ����ֲ����ϸ���ӽ�����������ϸ���ںϼ�������Ӧ�õ����֪ʶ��ʶ�Ǻ������������Լ�ʶͼ����������
(1) ֲ����ϸ���ӽ��ĵڢٲ���ȥ��ϸ���ڣ���þ��л�����ԭ�����塣��ֲ��ϸ���ڵ���Ҫ�ɷ�����ά�غ�����������õ�ȥ��ϸ���ڵķ�����ø�ⷨ����ʹ����ά��ø����ø����ϸ����
(2) �ڹ���Ϊ�˹��յ�ԭ�������ںϡ��յ��ںϵ����������������ġ����缤�ȣ���ѧ����һ�����þ��Ҷ�����PEG����Ϊ�յ������յ�ϸ���ںϡ��յ�����ϸ���ںϵķ������յ�ֲ��ԭ�������ںϵķ������ƣ�����ͬ�����յ�����ϸ���ںϻ����õ����IJ�����Ϊ�յ�����
(3) �۹������ںϵ�ԭ������������ϸ���ڣ����߶�������ֲ��ϸ���ڵ��γ��йء��ɼ�����ϸ���ڵIJ�����ϸ���ڸ߶�����������ء�
(4) �ܢݹ��̷ֱ��ʾ�ѷֻ����ٷֻ�����˿���ѵ���Ҫ�����ǣ��״�ϸ����Ⱦɫ�徭���ƺ�ȷ��ƽ�����䵽������ϸ����ȥ��
(5) ֲ����ϸ���ӽ������ֹ����е�ͻ���ŵ��ǿ��Կ˷�ԶԵ�ӽ����ϵ��ϰ�������ϸ���ںϼ�������Ҫ����;���Ʊ�����¡���塣

 ��������ϵ�д�
��������ϵ�д�