��Ŀ����
( 16�� ) ��ͼֻ��ʾ��ij��̬ϵͳ�ġ�����������ͼ�⣬���ͼ�ش��������⣺������š����ֻ���ĸ�ش�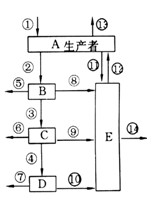
��1��ͼ�в�Ӧ�ô��ڵļ�ͷ�� ��
��2������������ͨ���������÷ֽ�һ�����л���ͷŵ������� ��
��3����B��������Ϊ100%��������Ϊ36%��������Ϊ48%����B��ͬ����Ϊ ��
��4������һ������ר��B�ķ��Ϊʳ����B��ij��ʱ������ͬ��������Ϊ107ǧ�������ⲿ�������п������������ڵĴ�ԼΪ�� ��
| A��106ǧ�� | B��0ǧ�� | C��2��106ǧ�� | D��106��2��106ǧ�� |
��6��ͼ�����ﹹ�ɵ�ʳ������ ��Ӫ������
��7��C�е�������Լֻ��B�������� ��
��8���������̬ϵͳ�ܵ�DDT��һ���л���ũҩ����Ⱦ������DDT������ߵ������ǣ� ��
��1��12 ��2���� ��3��64% ��4��B ��5��11 ��6��4 ��7��10%��20% ��8��D
����

 ����С����ͬ������ϵ�д�
����С����ͬ������ϵ�д�(16��) ��ͼ��ijֲ��Ҷ��ϸ���й�����úͺ������õ����ʱ仯ʾ���ͼ�����Т١���Ϊ�������̣�a��h Ϊ�������ơ����ͼ�ش�
��1���١��ݹ����У��ܹ����� ATP �Ĺ����� ________(��ͼ������)�����̢ܷ����ij�����____________��
��2������ d �Ľṹ��ʽ��___________��
��3�����罫��ֲ��ӹ���������ͻȻ�Ƶ��ڰ��������ڶ�ʱ�������� f �ĺ�������_____________�����罫��ֲ��ĸ�ϸ�������ڸ��������Ļ����У�һ��ʱ���ϸ�����ܹ����еĹ�����_ ����
________________________����ͼ�����֣���
��4���о������֣��������ɺ�ʱ����ϸ����Ѹ�ٺϳ�ij�ֻ�ѧ����X�������Ʋ�����ϳɵ�X���䵽ҶƬ���ܵ������� ���ա�������������ʵ�飺��ͬһֲ���ϼ�ȡ��С������״̬һ�µ�3ƬҶ���ֱ�Ҷ���²����ڲ�ͬŨ��X������Һ�С�һ��ʱ���õ��й��������±���ʾ��(ע��������Խ���������̶�Խ��)
���ա�������������ʵ�飺��ͬһֲ���ϼ�ȡ��С������״̬һ�µ�3ƬҶ���ֱ�Ҷ���²����ڲ�ͬŨ��X������Һ�С�һ��ʱ���õ��й��������±���ʾ��(ע��������Խ���������̶�Խ��)
| ���� ����ָ�ꡡ���� | ����Һ��X��Ũ��/mol��m��3 | ||
| 5��10��5 | 5��10��4 | 5��10��3 | |
| ҶƬ��X��Ũ��/nmol��g��1(����) | 2.47 | 2.97 | 9.28 |
| ҶƬ��������/mol��m��2��s��1 | 0.54 | 0.43 | 0.27 |
�� ���Ϸ����в����Ƶĵط�����ָ����������������

��
������������Ϊ�������ƺ�õ��Ľ������ô���Ʋ⣬��һ����Ũ�ȷ�Χ�ڣ���������Һ��X��Ũ������ҶƬ��������ǿ��____________________��


 ��̥��ֲ������ѧ�����Ƕȿ���B�Ĵ���ʹ���鶯��� ��һ��ʱ���ڣ������� ������֯�ϵ���ϵ�����Ǵ�������״̬�����Ϊ �ṩ�˿��ܡ�
��̥��ֲ������ѧ�����Ƕȿ���B�Ĵ���ʹ���鶯��� ��һ��ʱ���ڣ������� ������֯�ϵ���ϵ�����Ǵ�������״̬�����Ϊ �ṩ�˿��ܡ� �� �����⣬���Ա���Ʒ��棬����Ӧ�ü�ֵ��������� �� �����ǵ�ԭ���ֱ��� ��PCR����ԭ���� ��
�� �����⣬���Ա���Ʒ��棬����Ӧ�ü�ֵ��������� �� �����ǵ�ԭ���ֱ��� ��PCR����ԭ���� ��