��Ŀ����
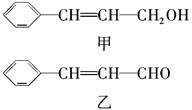
����Ŀ���л���W������ʽC3H6O3������NaHCO3��Ӧ�������к������ֻ�Բ�ͬ����ԭ�ӣ�������Ϊ3��1��1��1��
��1��W�Ľṹ��ʽ��_______________��
��2��W�ڲ�ͬ�����¿���ˮ�γɲ�ͬ���ʣ�
��������W��Ӧ�γ���״�����ṹ��ʽΪ_____________________��
��������W��Ӧ�γɻ�����д����Ӧ����ʽ��_____________________��
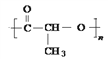
W�����γɸ߷��ӻ�����������ṹ��ʽ�ɱ�ʾΪ__________��
��3��W��ij��ͬ���칹������������ʣ�
�ܷ���������Ӧ��1mol�������ܸ������Ʒ�Ӧ����1molH2��
���ͬ���칹��Ľṹ��ʽΪ_______________________����֪ͬһ��̼ԭ���ϲ�������2���ǻ�����
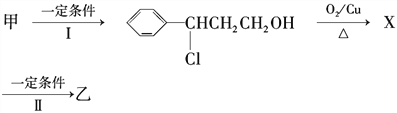
��4��W�Ĺ�ҵ�ϳ�·������ͼ��ʾ��

��֪����A��B��C��D��W�����к�����̼ͬԭ������
��![]()
���A������_________________��
��B������Cu(OH)2��Ӧ�Ļ�ѧ����ʽ��___________________��
��ij�л�����C��Ϊͬ���칹�壬���������࣬�ֺ�ȩ��������л�����ܵĽṹ��ʽΪ____________________ ��
���𰸡� ![]()
![]() 2
2 ![]()
![]()
 +2H2O
+2H2O 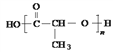 ��
��
![]() 1������ CH3CH2CHO��2Cu(OH)2
1������ CH3CH2CHO��2Cu(OH)2![]() CH3CH2COOH��Cu2O����2H2O HCOOC2H5
CH3CH2COOH��Cu2O����2H2O HCOOC2H5
�����������������������Ҫ�����л���Ľṹ�����ʡ�
��1��W�Ľṹ��ʽ��![]() ��
��
��2��W�ڲ�ͬ�����¿���ˮ�γɲ�ͬ���ʣ�
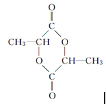
��������W��Ӧ�γ���״�����ṹ��ʽΪ![]() ��
��
��������W��Ӧ�γɻ�������Ӧ�Ļ�ѧ����ʽΪ��2 ![]()
![]()
 +2H2O ��
+2H2O ��
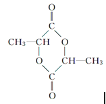
W�����γɸ߷��ӻ�����������ṹ��ʽ�ɱ�ʾΪ ��
�� ��
��
��3��W��ij��ͬ���칹���ܷ���������Ӧ��˵������ȩ����1mol�������ܸ������Ʒ�Ӧ����1molH2��˵�������ʷ����к��������ǻ������ͬ���칹��Ľṹ��ʽΪ![]() ��
��
��4�� ��A��1������ ��
��B�DZ�ȩ��B������Cu(OH)2��Ӧ�Ļ�ѧ����ʽ��CH3CH2CHO��2Cu(OH)2![]() CH3CH2COOH��Cu2O����2H2O ��
CH3CH2COOH��Cu2O����2H2O ��
��C�DZ��ᣬij�л�����C��Ϊͬ���칹�壬���������࣬�ֺ�ȩ����˵��C�Ǽ�����������л�����ܵĽṹ��ʽΪHCOOC2H5��

 ������ÿ�ʱ��ҵϵ�д�
������ÿ�ʱ��ҵϵ�д�