��Ŀ����
��09�ϲ���һģ����7�֣����������ںڰ����ȷ����ⶨ��ѿ�������и����������غ����������ͼA��ʾ�����ͼ�����ش�
![]()
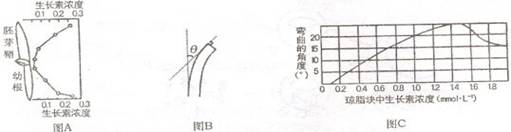
��1�����������ȷ������У��ϳ���������ʢ�IJ�λ��_______��_______��
��2�����ڸ����쳤��ϸ���쳤����������Դ��_______��
��3���г�������ѿ�ʵĶ��ˣ�Ȼ�����������ú��в�ͬŨ�������ص���֬�飬�����ںڰ���12h����ѿ�ʿ������������������Ƕ���ͼB��ʾ��������Ũ���������Ƕȹ�ϵ��ͼC ��ʾ��ʵ��Ҫ�ںڰ��н��е�ԭ����_________________��
��4�������˵��ʵ������_________________��
�𰸣�
��1����ѿ�ʵļ�� ����
��2�����������
��3��������ն�ʵ�����ĸ���
��4�������ص�Ũ����0 mol?L1��1.5 mol?L1��Χ�ڣ�����Ũ�ȵ����ߣ�����ѿ�������Ĵٽ�������ǿ����1.5 mmol?Lʱ���ٽ����������1.5 moI?L1ʱ���ٽ�����������

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д���09�ϲ���һģ����15�֣���Ӭ��һ��С��Ӭ�࣬�ɳ�����ֻ��0.6���ף���ͬ�������С��
����ͨ��ӬС�öࡣϲ���ڸ��õ�ˮ���ͷ��������Χ���衣ÿ�����ھ��ܷ�ֳ���µ�һ�����ǽ����Ŵ�ʵ��ġ����ǡ����ϡ��±��ǹ�Ӭ�����������״���ӽ�ʵ���¼��������ش�
| �� | �� | ||
�� | �� | �� | �� | |
P | ��CO2ֱ��ë���� | CO2���о���ë���� | CO2���о���ë���� | ��CO2ֱ��ë���� |
F1 | ��CO2ֱ��ë���� | ��CO2ֱ��ë���� | CO2����ֱ��ë���� | CO2���о���ë���� |
F2 |
| �磺CO2����ֱ��ë���� | �磺CO2����ֱ��ë���� |
|
��1�����������ӽ���ʽ���Ŵ�ʵ���л���Ϊ_____________��
��2����ӬCO2���������ë��״���������״�Ƿ����������϶���?______��ԭ���� ________________��
��3�������һ���Ŵ�ͼ�����������һ���ӽ�ʵ�飬�����ж���Ӭ��ɫ������A��a��ʾ����_______Ⱦɫ���ϡ�
�Ŵ�ͼ�⣺________________________________________________________________��
��4����F1�����۹�Ӭ��F1���дƹ�Ӭ�ӽ��õ�F2�뽫F2�����Ͳ����ڱ�����Ӧ�ո�
��5�������ӽ�F2��CO2����ֱ��ë�����۹�Ӭռ_______��
��6����֪��Ӭ��һ��������״�ɵ�����h���ƣ�����֪���Ƹ���״�Ļ���h��λ�ں���Ⱦɫ���ϣ��������һ���ĵ��鷽�����е��飬��Ԥ���������
��������Ѱ�Ҿ��и�������״�Ĺ�Ӭ���е���
��________________________________________________________________��
�������________________________________________________________________��
��________________________________________________________________��
��________________________________________________________________��