��Ŀ����
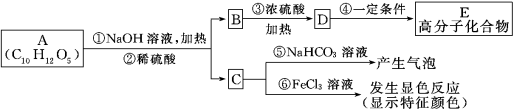
����Ŀ����ͼ��8���л��������ת����ϵ:

��ش���������:
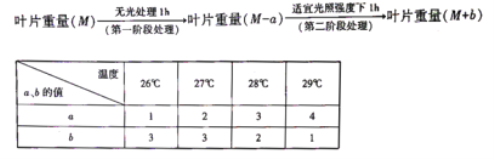
��1��A�����ʽΪ ��A�ĺ˴Ź��������� ��壬������֮��Ϊ ������ϵͳ������,������A�������� ��
��2��������ͼ��,���� ��Ӧ,���� ��Ӧ��(�Ӧ����)
��3��д����D����E�Ļ�ѧ����ʽ: ��
��4��C1�Ľṹ��ʽ�� ;F1�Ľṹ��ʽ�� ��
��5������8�ֻ�������,���ڶ�ϩ������ ,����A B C1 C2 D E F1 F2 ����ĸ���ţ���
��6��ֻ����һ��̼̼������E��ͬ���칹���� �֡�
���𰸡���1��C3H7��2��6:1��2,3-����������2��ȡ�����ӳ�
��3��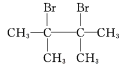 +2NaOH
+2NaOH![]()
![]() +2NaBr+2H2O
+2NaBr+2H2O
��4��(CH3)2C��C(CH3)2��![]() ��5��E��6��7
��5��E��6��7
��������
�����������1������A���ṹ��ʽ��֪A�����ʽΪC3H7��A�ĺ˴Ź���������2��壬������֮��Ϊ6:1������ϵͳ��������������A��������2��3-�������飻
��2������������ͼ�У�2��3-����������Cl2�ڹ���ʱ����ȡ����Ӧ������2��3-����-2-��-��������������ȡ����Ӧ��2��3-����-2-��-������NaOH���Ҵ���Һ�У��ڼ���ʱ������ȥ��Ӧ�γ�(CH3)2C=C(CH3)2��(CH3)2C=C(CH3)2��Br2�����ӳɷ�Ӧ�γ�D: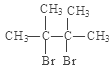 �����Է�Ӧ���Ǽӳɷ�Ӧ��
�����Է�Ӧ���Ǽӳɷ�Ӧ��
��3��������D ��NaOH���Ҵ���Һ�ڼ���ʱ������ȥ��Ӧ���γɻ�����E
��NaOH���Ҵ���Һ�ڼ���ʱ������ȥ��Ӧ���γɻ�����E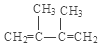 ��E����Ҫ�Ĺ�ҵԭ�ϣ�D����E�Ļ�ѧ����ʽ��
��E����Ҫ�Ĺ�ҵԭ�ϣ�D����E�Ļ�ѧ����ʽ��
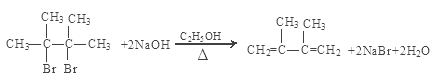 ��
��
��4��������2��������֪C1�Ľṹ��ʽ��(CH3)2C=C(CH3)2 ��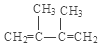 ��Br2����1��4�ӳ��γ�F1�Ľṹ��ʽ��
��Br2����1��4�ӳ��γ�F1�Ľṹ��ʽ��![]() ��
��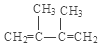 ��Br2����1��2�ӳ��γ�F2�Ľṹ��ʽ��CH2Br-CBr(CH3)-C(CH3)=CH2��
��Br2����1��2�ӳ��γ�F2�Ľṹ��ʽ��CH2Br-CBr(CH3)-C(CH3)=CH2��
��5������8�ֻ������У����ڶ�ϩ������E��
��6��ֻ����һ��̼̼������E��ͬ���칹����CH��CCH2CH2CH2CH3��CH3C��CCH2CH2CH3��CH3CH2C��CCH2CH3��CH��CCH2CH(CH3)2��CH��CCH(CH3)CH2CH3��CH3C��CCH(CH3)2��CH��CC(CH3)3������7����

 ��ս100��Ԫ����Ծ�ϵ�д�
��ս100��Ԫ����Ծ�ϵ�д�