��Ŀ����
����Ŀ��ͼ1�ǹ��ƺ��������ļ�����װ�á�����ͼʾ�ش��������⣺
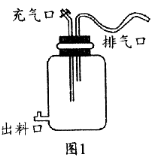
��1������֭װ�뷢��ƿǰҪ������ƿ��70%�ľƾ�������Ŀ���ǽ��� ������֭װ�뷢��ƿʱ��Ҫ���д�Լ1/3�Ŀռ䣬�ȿ�Ϊ ���ֿɷ�ֹ������ʢʱ����Һ�����
��2��ͼ1װ���еij������� ������Ҫ�رգ�������ܷ���ʹ����Һ����ķ�Ӧ��д���÷�Ӧ�ķ�Ӧʽ:
��3��д���ƾ������������������ƣ�
��4�������;��ֵ�ѡ����
��5����ĸϸ���̶����ɰܵĹؼ����������ƺ���������Һ������γɵ������鲻��Բ�λ���Բ�Σ�˵ �� �����ƹ�����ʹ��CaCl2��Һ�������� ��
���𰸡���1������ �ý�ĸ�������������������ٷ�ֳ����������
��2���ƾ����� C2H5OH+O2![]() CH3COOH+H2O
CH3COOH+H2O
��3���¶�Ϊ20-25��������
��4�������
��5����������Ũ��ƫ�ߣ�����ʧ�� ���ں������ƾ۳�
��������
�����������1������֭װ�뷢��ƿǰҪ������ƿ��70%�ľƾ�������Ŀ���ǽ���������ɱ���������֭װ�뷢��ƿʱ��Ҫ���д�Լ1/3�Ŀռ䣬�ȿ��ý�ĸ�������������������ٷ�ֳ�������������ֿɷ�ֹ������ʢʱ����Һ�����
��2��ͼ1װ���еij������ڽ�ĸ�����оƾ���������Ҫ�رգ���Ϊ��ĸ��Ҫ����״̬�²Ž����������������ƾ����������ɵ������ƾ������ڴ���������·��ͱ�ɴ��ᡣ�ƾ��ڴ���������±�ɴ���ķ�Ӧʽ:C2H5OH+O2![]() CH3COOH+H2O��
CH3COOH+H2O��
��3���ƾ���������Ҫ���Ƶ������������¶�Ϊ20-25�������������ȡ�
��4�������͵ľ����Ǵ������
��5����ĸϸ���̶����ɰܵĹؼ����������ƺ���������Һ������γɵ������鲻��Բ�λ���Բ�Σ�˵����������Ũ��ƫ�ߣ�����ʧ�ܡ����ƹ�����ʹ��CaCl2��Һ��������ʹ���������γɾ۳���
�������� | �������� | |
���� | ��ĸ�� | ����� |
����ԭ�� | ���������������������½��оƾ����� | ���������������Դ����ʱ�����Ƿֽ�ɴ����ȱ����Դʱ�����Ҵ���Ϊ��ȩ���ٽ���ȩ��Ϊ���� |
��Ӧʽ | C6H12O6��6O26CO2��6H2O C6H12O62C2H5OH��2CO2 | C6H12O6��2O2��2CO2��2H2O C2H5OH��O2��H2O |
���ʷ����¶� | 18 ����25 �� | 30 ����35 �� |
���������� | ǰ�ڣ����������ڣ������� | ������� |
pH | ���Ի�����4��0��5��8�� | ���Ի�����5��4��6��3�� |
����ʱ�� | 10��12 d | 7��8 d |

 ��У����ϵ�д�
��У����ϵ�д�