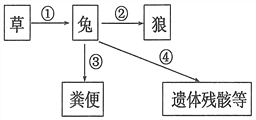
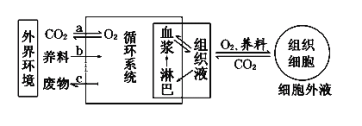
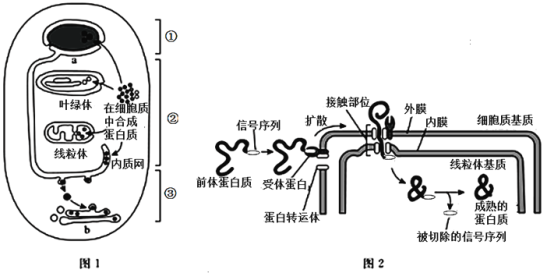
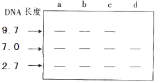
��Ŀ����
����Ŀ�������ʺ�������ʯ��ʯ�绯�����ĸƻ������У������ӻ���������ǿ����˳����ڿ��������ػָ������е���ֲ��Ϊ�������������ͺ��ԣ�ij���������ͺ������������У�����ͼ��ͼ��ʾ���ش��������⣺
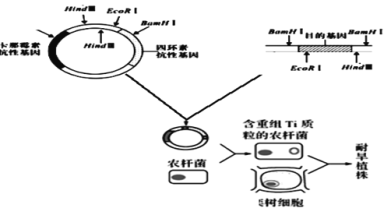
����ø | BamHI | Hind�� | EcoRI |
ʶ�����м��и�λ�� | G��GATCC CCTAG��G | A��AGCTT TTCGA��A | G��AATTC CTTAA��G |
��1���������������Ĺ����У�Ϊ�˱���Ŀ�Ļ���������и�������ĩ�˷����������ӣ�Ӧѡ������ø_______________�ֱ��и�����ͺ�Ŀ�Ļ����DNAƬ�Σ���ȫø�к������DNAƬ�ηֱ�Ϊ_____________����DNA����ø���Ӻ��Եõ�___________������������
��2��������ͺ�����ֱ�ӵ�������ϸ����һ������£�������������ͺ����ԣ�ԭ�����ͺ�����������ϸ���в���___________��Ҳ����___________��
��3�����λ���Ӧ���뵽ũ�˾�________________�ϣ�ͨ��ת�����ý�������ϸ�������ϵ�________������__________________________________�Ĺؼ���
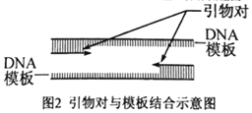
��4��Ҫ��ô��������λ�������PCR��������������ͼ2Ϊ�������ģ����ʾ��ͼ�������ĵ�5�Σ���Ҫ_________�������1Ϊ����ģ����Ƶ������������У����ж���һ������ɲ��ýϸߵ��˻��¶ȣ�________��
����� A | P P |
����� B | S S |
���𰸡�BamH���Hind�� 3��4 2 �ȶ����ڣ����ƣ� ���� Ti������T-DNA�� Ⱦɫ��DNA�� Ŀ�Ļ����ܷ�������ϸ�����ȶ��Ŵ��Ĺؼ� 32 �����B
��������
������ͼ����Ŀ�Ļ����DNA�������Ͼ���Hind����BamH����EcoR��ø��λ�㣬��EcoR��ø��λ��λ��Ŀ�Ļ����ϣ��и���ƻ�Ŀ�Ļ���BamH��ø�и�����������������BamHI ��Hind��ø����ԴDNA�����������и
��1���ɷ�����֪��Ϊ�˱���Ŀ�Ļ���������и�������ĩ�˷����������ӣ���Ҫʹ�� Hind���BamH����Ti�����Ͻ����и��������2��Hind���ø��λ�㣬��1��BamH���ø��λ�㣬��ȫø�к������ϵ�DNAƬ��Ϊ3��Ŀ�Ļ�������һ��Hind�������BamH���ø��λ�㣬��ȫ�и�������DNAƬ��Ϊ4��ʹ��ʹ�� Hind���BamH��ø�к�Ŀ�Ļ�����2��Ƭ�ξ��������������ӣ���˾�DNA����ø���Ӻ��Եõ�2������������
��2��������ͺ�����ֱ�ӵ�������ϸ����һ������£�������������ֲ�겻�����ͺ����ԣ�ԭ�����ͺ�����������ϸ���в��ܸ��ƺͱ��������Ҫ��������������塣
��3���ͺ�����Ӧ���뵽ũ�˾�Ti������T��DNA�ϣ�ͨ��ת�����ý�������ϸ�������ϵ�Ⱦɫ��DNA�ϣ�ʹ�ͺ��������ȶ�ά�ֲ��������ã���Ҳ��Ŀ�Ļ����ܷ�������ϸ�����ȶ��Ŵ��Ĺؼ���
��4������4�Σ�����16��DNA���ӣ�DNAΪ˫������������5����Ҫ32������˻��¶ȱ����������ļ���������������趨�������B��G/C��������ϸߣ��ɲ��ýϸߵ��˻��¶ȡ�

 ��У����ϵ�д�
��У����ϵ�д�