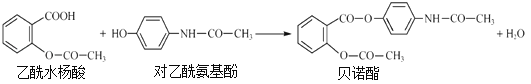
��Ŀ����
����Ŀ������ϩ�������л��ϳɡ���ͨ���������̣��Ի���ϩΪԭ�Ϻϳɻ��ѡ�������������F��������ȼ���Ŀ�������J�������ޱ���̼ԭ�ӡ�
��֪��R1-CH=CH-R2 ![]() R1-CHO+R2-CHO
R1-CHO+R2-CHO

(1)�۵ķ�Ӧ������_____________��
(2)H��������_____________ ��
(3)�л���B��I�Ĺ�ϵΪ___________ (����ĸ)��
A.ͬϵ�� B.ͬ���칹�� C.�����ڴ��� D.��������
(4)д�����з�Ӧ�Ļ�ѧ����ʽ��ע����Ӧ����:
��Ӧ��_______________��_________ ��
��Ӧ��_______________��__________��
��Ӧ��_______________��__________ ��
��Ӧ��________________��__________ ��
(5)д������D�������������״ͬ���칹��Ľṹ��ʽ��_________��_________��
���𰸡��������Ƶ��Ҵ���Һ������ ���Ҷ����Ҷ��� C 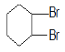 +2NaOH
+2NaOH![]()
 +2NaBr ȡ����Ӧ nHOOC-COOH+nHOCH2CH2OH
+2NaBr ȡ����Ӧ nHOOC-COOH+nHOCH2CH2OH![]()
![]() +(2n-1)H2O ���۷�Ӧ HOCH2CH2CH2CH2OH
+(2n-1)H2O ���۷�Ӧ HOCH2CH2CH2CH2OH![]() CH2=CHCH=CH2+2H2O ��ȥ��Ӧ nCH2=CHCH=CH2
CH2=CHCH=CH2+2H2O ��ȥ��Ӧ nCH2=CHCH=CH2 ![]()
![]() �Ӿ۷�Ӧ CH2=CHCOOCH3 CH3COOCH=CH2
�Ӿ۷�Ӧ CH2=CHCOOCH3 CH3COOCH=CH2
��������
����ϩ����ˮ�����ӳɷ�Ӧ����A����A�Ľṹ��ʽΪ ��A��NaOHˮ��Һ����ȡ����Ӧ����B����B�Ľṹ��ʽΪ
��A��NaOHˮ��Һ����ȡ����Ӧ����B����B�Ľṹ��ʽΪ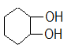 ��
��![]() ��
��![]() �����·�����֪������Ӧ���ϼ���������D��C������G��Cԭ�������ж�D��C��Cԭ��������D�к�4��Cԭ�ӣ��ṹ��ʽΪOHC-(CH2)2-CHO��C�к�2��Cԭ�ӣ�C�Ľṹ��ʽΪOHC-CHO��C�ڴ�������H2������Ӧ���ɴ�F��FΪ�Ҷ�����F�Ľṹ��ʽΪHOCH2CH2OH��C�ڴ�������O2������Ӧ��������E��EΪ�Ҷ��ᣬE�Ľṹ��ʽΪHOOC-COOH��E��F�ڴ��������·���������Ӧ���ɾ��Ҷ����Ҷ���H����H�Ľṹ��ʽΪ
�����·�����֪������Ӧ���ϼ���������D��C������G��Cԭ�������ж�D��C��Cԭ��������D�к�4��Cԭ�ӣ��ṹ��ʽΪOHC-(CH2)2-CHO��C�к�2��Cԭ�ӣ�C�Ľṹ��ʽΪOHC-CHO��C�ڴ�������H2������Ӧ���ɴ�F��FΪ�Ҷ�����F�Ľṹ��ʽΪHOCH2CH2OH��C�ڴ�������O2������Ӧ��������E��EΪ�Ҷ��ᣬE�Ľṹ��ʽΪHOOC-COOH��E��F�ڴ��������·���������Ӧ���ɾ��Ҷ����Ҷ���H����H�Ľṹ��ʽΪ![]() ��F��ŨH2SO4���������ɻ���G����G�Ľṹ��ʽΪ
��F��ŨH2SO4���������ɻ���G����G�Ľṹ��ʽΪ ��D��H2�ڴ��������·����ӳɷ�Ӧ���ɶ�����I����I�Ľṹ��ʽΪHOCH2CH2CH2CH2OH��I��ŨH2SO4�����·�����ȥ��Ӧ����1��3-����ϩJ����J�Ľṹ��ʽΪCH2=CHCH=CH2��J��һ�������·����Ӿ۷�Ӧ�ϳ����ṹ��ʽΪ
��D��H2�ڴ��������·����ӳɷ�Ӧ���ɶ�����I����I�Ľṹ��ʽΪHOCH2CH2CH2CH2OH��I��ŨH2SO4�����·�����ȥ��Ӧ����1��3-����ϩJ����J�Ľṹ��ʽΪCH2=CHCH=CH2��J��һ�������·����Ӿ۷�Ӧ�ϳ����ṹ��ʽΪ![]() ���ݴ˽��
���ݴ˽��
(1)AΪ ������
������![]() ��±��������ȥ��Ӧ����Ӧ����Ϊ�������Ƶ��Ҵ���Һ�����ȣ���Ϊ�������Ƶ��Ҵ���Һ�����ȡ�
��±��������ȥ��Ӧ����Ӧ����Ϊ�������Ƶ��Ҵ���Һ�����ȣ���Ϊ�������Ƶ��Ҵ���Һ�����ȡ�
(2)H�ĽṹΪ![]() ����������Ϊ���Ҷ����Ҷ�������Ϊ���Ҷ����Ҷ�����
����������Ϊ���Ҷ����Ҷ�������Ϊ���Ҷ����Ҷ�����
(3)�л���BΪ ��IΪHOCH2CH2CH2CH2OH�����Ƿ���ʽ��ͬ��̼���ṹ��ͬ���������ж������ǻ������ڴ��ࣻ��ΪC��
��IΪHOCH2CH2CH2CH2OH�����Ƿ���ʽ��ͬ��̼���ṹ��ͬ���������ж������ǻ������ڴ��ࣻ��ΪC��
(4)��Ӧ�ڣ�����A����B����ѧ����ʽΪ +2NaOH
+2NaOH![]()
 +2NaBr����Ӧ����Ϊȡ����Ӧ����Ϊ
+2NaBr����Ӧ����Ϊȡ����Ӧ����Ϊ +2NaOH
+2NaOH![]()
 +2NaBr��ȡ����Ӧ��
+2NaBr��ȡ����Ӧ��
��Ӧ�࣬E��F��Ӧ����H����ѧ����ʽΪnHOOC-COOH+nHOCH2CH2OH![]()
![]() +(2n-1)H2O����Ӧ����Ϊ���۷�Ӧ����ΪnHOOC-COOH+nHOCH2CH2OH
+(2n-1)H2O����Ӧ����Ϊ���۷�Ӧ����ΪnHOOC-COOH+nHOCH2CH2OH![]()
![]() +(2n-1)H2O�����۷�Ӧ��
+(2n-1)H2O�����۷�Ӧ��
��Ӧ�ᣬ����I����J����ѧ����ʽΪHOCH2CH2CH2CH2OH![]() CH2=CHCH=CH2+2H2O����Ӧ����Ϊ��ȥ��Ӧ����ΪHOCH2CH2CH2CH2OH
CH2=CHCH=CH2+2H2O����Ӧ����Ϊ��ȥ��Ӧ����ΪHOCH2CH2CH2CH2OH![]() CH2=CHCH=CH2+2H2O����ȥ��Ӧ��
CH2=CHCH=CH2+2H2O����ȥ��Ӧ��
��Ӧ�⣬����Jͨ���Ӿ۷�Ӧ�ϳ�����ѧ����ʽΪnCH2=CHCH=CH2 ![]()
![]() ����Ӧ����Ϊ�Ӿ۷�Ӧ����ΪnCH2=CHCH=CH2
����Ӧ����Ϊ�Ӿ۷�Ӧ����ΪnCH2=CHCH=CH2 ![]()
![]() ���Ӿ۷�Ӧ��
���Ӿ۷�Ӧ��
(5)D�Ľṹ��ʽΪOHC-(CH2)2-CHO������ʽΪC4H6O2�������������״ͬ���칹��ΪCH2=CHCOOCH3��CH3COOCH=CH2����ΪCH2=CHCOOCH3��CH3COOCH=CH2��