��Ŀ����
2009��ŵ��������ѧ��ҽѧ���������ֶ����Ͷ���ø��α���Ⱦɫ�����λѧ�ߡ����������ϸ����Ⱦɫ��ĩ�˵�DNA�ظ�Ƭ�Σ����������Ⱦɫ��ĩ�˵ĵĸ��ƣ���ֹȾɫ���ںϡ�����ͽ��⡣������ϸ������ʱ���ܱ���ȫ���ƣ��������Ѵ��������Ӷ����̣������ж���ø�Ĵ��ڡ�����ø��Ҫ�ɷ���RNA�͵����ʣ��京����������ʶ��λ�㣬��������RNAΪģ�壬�ϳɶ���DNA���ӵ�Ⱦɫ��ĩ�ˣ�ʹ�����ӳ����Ӷ��ӳ�ϸ������������ʹ�����������о����������ϸ���в����ڶ���ø�Ļ��ԣ�Ⱦɫ�彫��ÿ�η��Ѷ����Խ��Խ�̣���������ϸ���ĺ����������Ķ�ʧ������������
��1��Ⱦɫ��ĩ�˷��������ں�����Ⱦɫ��ṹ�����е� �����ʹȾɫ���ϻ���� �����ı䡣
��2������ø�У������ʳɷֵĹ��������� ø������������������άϸ����ϸ���еĶ���������ϸ����ֳ������ ������ء�����أ���ϵ��
��3���о���Ա��С������һ����Ⱦɫ���Ͽ��ƶ���ø����B�ó���������һֻ������ΪB+B��������С��B+��ʾ����B����B����ʾȥ����B������������ֻ����С��ѡ��B��B������С��������B+B��С������ױ��� ���ӽ��Ӵ��Ļ������� ��F1���д���С��������䣬�Ӵ��з�������Ҫ��ĸ���ĸ����� ��
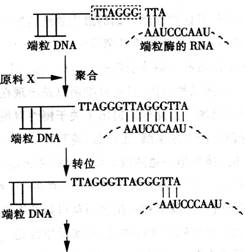
��4������Ϊ��������ϸ�����������ʾ��ͼ��
![]()
��ͼ��������ȷ���� ��
A���ٹ��̱���ϸ�������������Եģ�����������Ĥ����Ϣ���ݹ���
B��ϸ���������������µ����ʺϳɣ������˻����ѡ���Ա���
C���ڹ����е���ϸ�������ɣ�����ϸ��������ϸ��������������
D��������ػ����ǻ�����еģ��ڶ����������������з�����Ҫ����
��5���Ŵ�ѧ�Ͻ�Ⱦɫ����ijһ���μ�����еĻ���һ��ʧ�������ȱʧ����һ��ͬԴȾɫ��������Ⱦɫ������ͬ����ͬʱȱʧ��ȱʧ�����ӣ�����һ��Ⱦɫ�巢��ȱʧ����һ��������ȱʧ�Ӻ��ӡ�ȱʧ�Ӻ��ӵ����������͵��ܴ�ȱʧ�����ӳ����¸���������
����һ���۹�ӬXAY��һ���۴ƹ�ӬXaXa�ӽ����Ӵ��г���һֻ���۴ƹ�Ӭ����������ַ����ж���ֻ���۴ƹ�Ӭ�ij���������ȱʧ��ɵģ��������ڻ���ͻ������ģ�
����һ��
��������
��22�֣���1����λ ������˳��
��2����ת¼ �����
��3��B+ B+������ B+ B+��B+B- 1/32
��4��ABD
��5������һ��ȡ�ù�Ӭ�з���������ϸ���Ƴ�װƬ�������¹۲�Ⱦɫ��ṹ����Ⱦɫ�������������ǻ���ͻ������ģ���֮���ܿ�����Ⱦɫ��ȱʧ��ɵġ�
��������ѡ�ð��۴ƹ�Ӭ������۹�Ӭ�ӽ������ӽ��Ӵ��дƹ�Ӭ�����۹�Ӭ����Ϊ1��1������ֻ���۴ƹ�Ӭ�ij��������ڻ���ͻ������ģ����ӽ��Ӵ��дƹ�Ӭ�����۹�Ӭ����Ϊ2��1������ֻ���۴ƹ�Ӭ�ij���������ȱʧ��ɵġ�
����:
��1��Ⱦɫ�巢�������ںϵ��·�ͬԴȾɫ���Ľṹ�����ı䣬����Ⱦɫ��ṹ�����е���λ��Ⱦɫ��ṹ����ʹȾɫ���ϵĻ������Ŀ������˳�����ı䣬������������״�ĸı䡣
��2���������֪�������ƾ�����RNAΪģ��ϳ�DNA�Ĺ��ܣ�����书����������ת¼ø�����Ĵ�����������Ⱦɫ������ij��̣��Ӷ�������ϸ����ֳ�Ĵ�����
��3������ԭ����С���еĻ�����ֻ��B+B+��������B+B-������С����B-B-�Ĵ���С����ȻΪ��B+B-���ᣩ��B+B+���⣩��F1��B+B-��B+B+����F1�д���С�����������F2����B-B-����С��F1�Ĵ�����С��Ļ����;�ΪB+B-��B+B+�ұ�����ȣ����F1������������F2��B-B-����С��ı���=1/2��1/2��1/4��1/2=1/32��
��4���ɻ�����������ϸ���Զ����������Ĺ��̣���ϸ�����������ǵ����յ�������Ĥ���巢�������Խ�ϣ�����ϸ���������������ԣ�����������Ĥ����Ϣ���ݹ��ܣ�ϸ���������������µ����ʺϳɣ������˻����ѡ���Ա���ڹ����е���ϸ�������ɣ���ϸ�����������Ĺ��̣�������ػ����ڻ����б����ʹ��ڣ��ǹ��еģ��ڶ����������������еIJ�ͬʱ�ڱ���Ӷ�������Ҫ���á����C����ȷ��
��5������һ�����������ķ����۲�ù�Ӭ���ѵ�ϸ����Ⱦɫ��ṹ�Ƿ����ı䣬ȷ���ñ��������Ի���ͻ�仹��Ⱦɫ��ȱʧ��ɵġ�
�����������������Ϣ��ȱʧ�����ӵ��¸���������һ���ɽ��ù�Ӭ������۹�Ӭ�ӽ����۲첢ͳ���Ӵ������Ը�����Ŀ�ı�����ȷ���ñ������Դ����Ϊ����ͻ�䣬��XaXa��XAY��XAXa��XaY���Ӵ����۱����൱�����ñ���ΪȾɫ��ȱʧ��ɣ���XaX��XAY��XAXa��XAXa��XaY��XY�����������Ӵ����۱���Ϊ2��1��

 ��У����ϵ�д�
��У����ϵ�д�