��Ŀ����
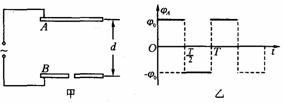
��ͼ����ʾ��A��B���������ˮƽ���ã����Ϊd=0. 6 cm����������һ�����Ա仯�ĵ�ѹ����B��ӵأ�![]() ��ʱ��A�����
��ʱ��A�����![]() ����ʱ��仯�������ͼ����ʾ������һ�����������t=0ʱ�̴�B������С������糡�����ô������ܵ��ĵ糡��Ϊ������������������糡ʱ���ٶȿɺ��Բ��ƣ���
����ʱ��仯�������ͼ����ʾ������һ�����������t=0ʱ�̴�B������С������糡�����ô������ܵ��ĵ糡��Ϊ������������������糡ʱ���ٶȿɺ��Բ��ƣ���
��1����0��![]() ��
��![]() ��T������ʱ�������ļ��ٶȴ�С�ͷ���
��T������ʱ�������ļ��ٶȴ�С�ͷ���
��2��Ҫʹ��������A�����������ӵ�ѹ�������Ϊ���٣�g=10 m/s2����
(1)��糡����СΪF����F= 2mg������t=0ʱ��
�����������ǰ��������ڣ���
![]() ���������ϣ�
���������ϣ�
�������ڵļ��ٶ�a2����
![]() ��������£�
��������£�
(2)ǰ�����������ĸ߶�![]() ��ǰ����������ĩ�ٶ�Ϊ
��ǰ����������ĩ�ٶ�Ϊ![]() ������������������ȼ����˶���������˶�ʱ��t1����3gt1=
������������������ȼ����˶���������˶�ʱ��t1����3gt1=![]() ��
��![]() ���˶�ʱ���������ĸ߶�
���˶�ʱ���������ĸ߶�![]() ��
��
���������ܸ߶�Ϊ![]() ��
��
������ڵ�![]() ʱ���ڣ������¼����˶����½��ĸ߶�
ʱ���ڣ������¼����˶����½��ĸ߶�![]() ��
��
�����������������һ�������ڵ���λ��Ϊ�㣬ֻҪ�����������в���A���������ɣ���![]() ����
����![]() .
.
���ӵ�ѹ�������Ϊ![]() ��
��
����:
��

��ϰ��ϵ�д�
 �п������п��Ծ����ϵ�д�
�п������п��Ծ����ϵ�д�
�����Ŀ
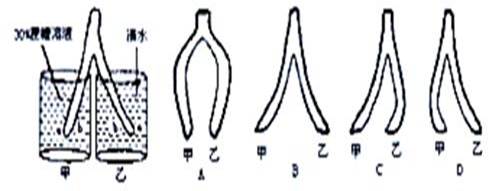
 ϸ���ڱ�������չ���ж�30 min����ܳ��ֵ���״��________��
ϸ���ڱ�������չ���ж�30 min����ܳ��ֵ���״��________��