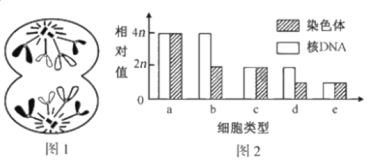
��Ŀ����
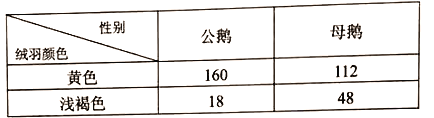
����Ŀ��ʵʩ�������ս���ǽ��������й��Ĺؼ��ҹ��������һ�Ķ���ֳ�����Լռ�����������90%������ʶ��շ����ij����Ա𣬶Թ�ģ����ֳ������Ҫ��ij��Ʒ�ּշ��������ij�������ͨ���ʻ�ɫ��dz��ɫ������9���º����Գ��죬�����˾�������������ɫ���ǰ�ɫ����ѧ���ڷ��������У�ѡ�����(10����)����40ֻ��ĸ��200ֻ�����л�Ⱥ������������䣬�������1�����εij���(G1)���۲�����������Ա�ͳ�ƽ�����¡���ش�
(1)ͬһֻ��ij����������ɫ��������ɫ�кܴ���죬��˵��������ɫ�Ļ���___________����ë�IJ����ǻ���ѡ���Ա���Ľ����
(2)�ݱ��Ʋ⣬��Ʒ�ּij���������ɫ___________Ϊ������״���Ʋ�������ɫ�Ŵ�������ǰ����Ŵ���������___________��
(3)Ϊ��һ��ȷ����Ʒ�ּ�������ɫ���Ŵ���ʽ���Ӹ����ΰ�(G1)��ѡȡ����������ɫΪ___________�Ĺ����___________��ĸ������ӽ�ʵ�飬ͨ����һ��F1������ɫ���Ա����ȷ�ϣ���F1��___________����˵����������ɫ�Ļ���ֻ��ZȾɫ���ϣ����ܿ��ٷֱ�����Ա�
(4)�����Ϸ�����ȷ����1����(G1)��ĸ���У�dz��ɫ����Ļ���Ƶ����___________������������___________��
���𰸡���ͬ����ֹһ�ԡ������ԡ����������ϡ��� ��ɫ ������ɫ���Ӵ������ĸ���г��ֲ��죬����������ɫ��صĻ������Ա������ dz��ɫ ��ɫ ���������Ϊ��ɫ��ĸ�������Ϊdz��ɫ 0.3 ��ĸ����dz��ɫ�Ļ���Ƶ�ʵ���dz��ɫĸ��Ļ�����Ƶ�ʣ���ĸ���У�ZȾɫ���Ͽ���dz��ɫ���������ռ����ĸ��������������ɫ��ػ��������ı�����
��������
�жϻ����λ�õķ�������һ�������֪�����ԣ�ȷ�������λ�õķ�����ѡ��ͬ��Ϊ���Ը��塢����Ϊ���Ը�����ӽ��������Ӵ��ı������жϡ�
������δ֪������ʱ��ȷ������λ��λ�ã���1����֪�Ӵ�����״��������ijһ��״���Ӵ��⡢�������ֵı��������������⡢������б���һ����Ϊ��Ⱦɫ�壻��ijһ��״�ڡ⡢���б�����ͬ����Ϊ�����Ŵ�����2����֪�״�����״����������������Ӵ�������һ�£���Ϊ��Ⱦɫ���Ŵ����Ӵ�������ֳ�����״���ױ�Ϊ�����ӣ����Ӵ������б����ߵ���״Ϊ���ױ��д������Ӻ��Ӷ����ڣ�Ϊ������״�����Ӵ������������һ�£������Ա���죬��ΪXȾɫ���Ŵ������Ӵ����۸���һ����״���ӽ�����������ױ����ֳ�����״Ϊ������״��
��1���������⣬ͬһֻ��ij����������ɫ��������ɫ�кܴ���죬��˵������״������Ǽ�һһ��Ӧ��ϵ������״�����������Ի��������ϵĵ�λ������Ƶģ���ë�IJ����ǻ���ѡ���Ա���Ľ����
��2����������ͱ������ݿ�֪����Ʒ�ּ��ӽ��ĺ����ĸ��ij���������ɫ��ɫ��dz��ɫ��Լ�ӽ�3:1����˵������������ɫ�еĻ�ɫΪ������״�����������֪�������ں����������ɫ���Ӵ������ĸ���г��ֲ��죬�����������ɫ��صĻ���������Ա��������
��3��Ҫȷ����Ʒ�ּ�������ɫ���Ŵ���ʽ�����жϻ����λ�ã�����֪�����Ե������£���ѡ��ͬ��Ϊ���Ը��塢����Ϊ���Ը�����ӽ�����ѡ��dz��ɫ�������ɫĸ���ӽ���ͨ����һ��F1������ɫ���Ա����ȷ�ϡ�����������ɫ�Ļ���ֻ��ZȾɫ���ϣ����Ӵ�����ȫΪ��ɫ��ĸ��ȫΪdz��ɫ��
��4�����ݱ������ݿ�֪����1����(G1)��ĸ���У�dz��ɫ�����ĸ��ռ48����48+112��=0.3�����ڣ�3���ʷ�����ȷ�������Ƹ���״�Ļ���ֻλ��ZȾɫ���ϣ����ݰ����Ŵ����ص㣬���1����(G1)��ĸ���У�dz��ɫ�Ļ���Ƶ�ʵ���dz��ɫĸ��Ļ�����Ƶ�ʣ����Ӵ�ĸ����dz��ɫ����Ļ���Ƶ����0.3��

����Ŀ��������Աʵ��̽����ɣҶ���Ƕ��˹�����С��ո�Ѫ����ֵ��Ӱ�죬ʵ�����������±���ʾ����д��ʵ��˼·�������һ������ϵ��������ʵ������������ֵ�仯����������ͼ��ʾ������
(˵�������иոմ����õ����˹�����С������ֻ����Ʒ��С��������ո�Ѫ����ֵΪ6.0 mmol/L����ҩ�Ͳⶨ�ո�Ѫ����ֵ�ķ�������Ҫ��ʵ�����6��ʱ�䡣)
ɣҶ���Ƕ��˹�����С��ո�Ѫ����ֵ��Ӱ��
ʵ����� (������ ��ҩ��) | ��ҩǰ �ո�Ѫ�� (mmol/L) | ��ҩ��ո�Ѫ��(mmol/L) | ||
2��ĩ(�� ��ҩǰ�Ƚ�) | 4��ĩ(�� 2��ĩ�Ƚ�) | 6��ĩ(�� ��ҩǰ�Ƚ�) | ||
A�� (0 g/kg) | 17.7 | �������� | �������� | ��������(�ӽ�2��ĩ��ֵ) |
B�� (0.25 g/kg) | 17.5 | �������� | �������� | ���������� |
C�� (0.50 g/kg) | 17.4 | �������� | ���������� | ���Խ���(���Ը���������ֵ) |
D�� (1.00 g/kg) | 17.3 | ���������� | ���������� | ���Խ���(�ӽ�������ֵ) |
(1)ʵ��˼·�� ____________________________________
(2)���һ������ϵ��������ʵ������������ֵ�仯����������ͼ��ʾ������
__________________