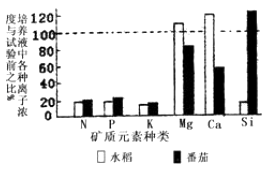
��Ŀ����
����Ŀ�������ϸ���ṹ������ص�֪ʶ�ش��������⣮ 
��1��������Σ�����ཡ����ɱ��֮һ��������������������կ����������Ű���֣����������ֽṹ�У����п��ܱ�ʾ�����������A������B����C�������жϵ���������û�� ��
��2��C�п�������ɫ��ϸ����������ţ���C�к��к����ϸ���ṹ��������ţ���AC���е�ϸ����Ϊ��ϸ�������ƣ���
��3��ϸ���ǽ���������Ļ�����λ��C��ͨ����������ˮ����ʧˮ��ԭ���������൱�ڰ�Ĥ��ֲ��һ�㲻�ʺ����μ������������Ҫԭ���� ��
��4������ϸ��Ҳ���Է��������ã�0.9%��NaCl��Һ�����õ���Һ��ѹ��ȵ�������ˮ��ijͬѧ����������״̬�µ��õ�һ���ſ�С�������ɼס��ҡ�������4��������ͨ��������ȵij�����ѪҺѭ����������������ʵ�飬ʵ�鲽��ͽ�������
ʵ����� | �� | �� | �� | �� |
����ע���������Һ��ʹ���ӯ | 0.7%NaCl | 0.9%NaCl | 1.1%NaCl | 0.9%NaCl+��Na+���嵰�����Ƽ���10mL |
ά���������°�Сʱ�⳦����NaCl��Һ���� | 1mL | 3mL | 5mL | 9.9mL |
ʵ�鿪ʼʱ��ˮ�ڳ�ǻ��ѪҺ֮����ƶ������ǣ�����������������Һ������Һ�������������Ƚ���ʵ���������Ʋ�С��������Na+ʱ����ҪNa+���嵰�IJ��룮
���𰸡�
��1��B��ϸ���ṹ��ϸ��Ĥ��
��2���٢�٢ڢۢࣻ������
��3��ԭ���ʲ㣻�����ҺŨ��Զ����ϸ��ҺŨ�ȣ�ֲ��ϸ������Ϊ��ʧˮ���ȶ�����
��4����������Һ���Ҷ�
���������⣺��1��������Σ�����ཡ����ɱ��֮һ��������������������կ����������Ű���֣����������ֽṹ�У�Bû��ϸ���ṹ��������Dz�������2��C�п�������ɫ��ϸ�����Т�Ҷ����͢�Һ�ݣ�C�к��к����ϸ���ṹ������Ҷ���塢�������塢��ϸ���ˡ�������壮A��ԭ��ϸ����C�����ϸ����ԭ��ϸ�������ϸ�����е�ϸ����Ϊ�����壮��3�������ֲ��ϸ����ͨ����������ˮ����ʧˮ��ԭ������ԭ���ʲ���൱�ڰ�Ĥ��ֲ��һ�㲻�ʺ����μ������������Ҫԭ���������ҺŨ��Զ����ϸ��ҺŨ�ȣ�ֲ��ϸ������Ϊ��ʧˮ���ȶ���������4������ϸ��Ҳ���Է��������ã�0.9%��NaCl��Һ�����õ���Һ��ѹ��ȵ�������ˮ��ijͬѧ����������״̬�µ��õ�һ���ſ�С�������ɼס��ҡ�������4��������ͨ��������ȵij�����ѪҺѭ����������������ʵ�飬ʵ�鲽��ͽ�������
ʵ����� | �� | �� | �� | �� |
����ע���������Һ��ʹ���ӯ | 0.7%NaCl | 0.9%NaCl | 1.1%NaCl | 0.9%NaCl+��Na+���嵰�����Ƽ���10mL |
ά���������°�Сʱ�⳦����NaCl��Һ���� | 1mL | 3mL | 5mL | 9.9mL |
���������֪��0.9%��NaCl��Һʱ���õ���Һ��ѹ��ȵ�������ˮ��ʵ���鿪ʼʱ��������ע��0.7%NaCl��Һ������ѹ����Ѫ����ѹ����˼��鳦ǻ�е�ˮ������Һ�����෴����ѪҺ�е�ˮ���볦ǻ���ҺͶ��м����Լ��IJ�����Ƿ���롰��Na+���嵰�����Ƽ������Ƚ��ҺͶ���ʵ���������е���Һ������û�м��٣����Ʋ�С��������Na+ʱ����ҪNa+���嵰�IJ��룮
���Դ��ǣ���1��B��ϸ���ṹ��ϸ��Ĥ����2���٢ᣨ��һ�������֣����٢ڢۢࣨ��һ�������֣��������壨3��ԭ���ʲ� �����ҺŨ��Զ����ϸ��ҺŨ�ȣ�ֲ��ϸ������Ϊ��ʧˮ���ȶ�������4����������Һ���Ҷ�
�����㾫�����������⣬������Ҫ�˽�ԭ��ϸ�������ϸ������̬�ͽṹ����ͬ(ԭ��ϸ����ϸ����С����Ĥ�����ʣ�û�г��ε�ϸ���ˣ����ϸ����ϸ���ϴ��к�Ĥ���к��ʡ���������ϸ����)��

 ���ٴ���������ѧϰ����ѧ�ں����ν�ϵ�д�
���ٴ���������ѧϰ����ѧ�ں����ν�ϵ�д�