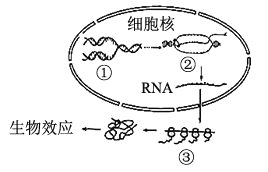
��Ŀ����
����Ŀ�����ͼ�ش������й���̬ϵͳ�����⣺
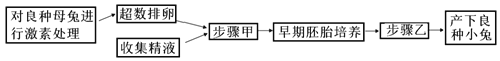
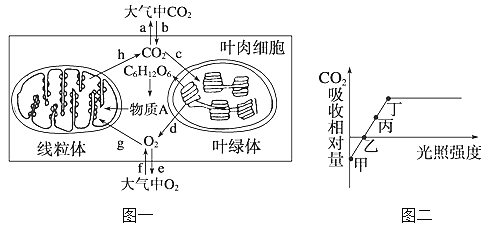
��1��ͼ������̬ϵͳ̼ѭ��ʾ��ͼ��ͼ��Dָ��A�ļ�ͷ����������������_____________________________��
��2����ͼ�ҵ�ʳ�����У�������________(����)��ͬ������һ���֡�
��3��ͼ�����ڵ���̬ϵͳ�У������ڵ�һӪ������������������δ���õIJ��ֺͷֽ��߷ֽ�����֮�⣬������������________________��________________��
��4����ͼ�ҵ�ʳ�����У���Ϊ���ṩ�˿��Բ�ʳ����Ϣ�����ܹ����������µ���ζȥ�Բ�����ͬ��Ҳ���������ǵ���ζ����Ϊ��������Բ�����Щ��Ϣ��������________________________����ά����̬ϵͳ���ȶ���
��5��ͼ����ʾ��̬ϵͳ������ӥ֮���õ����������ڷ����仯�����մﵽƽ�⣬�������̬ϵͳ�ڲ�����һ����____________��������ɭ����̬ϵͳ��ȣ���ԭ��̬ϵͳ________(�����ֿ����������ָ�����)�ȶ��Ը��ߡ�
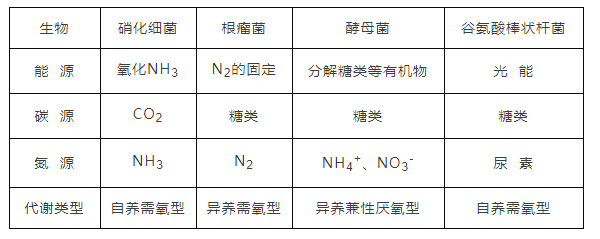
��6���±���ij��̬ϵͳ����Ӫ����(����һӪ����)������������[��λ��J/(hm2��a)]
Ӫ���� | ͬ���� | δ���� ���� | �ֽ��� �ֽ��� | ������ ���� |
A | 2.48��1011 | 2.00��109 | 1.20��1010 | 3.60��1010 |
B | 2.40��107 | 6.00��105 | 4.00��105 | 2.30��107 |
C | 7.50��108 | 2.40��107 | 6.00��106 | 7.16��108 |
�������̬ϵͳ����Ⱥ�����������________ J/(hm2��a)������̬ϵͳ�ڶ�Ӫ����������Ӫ��������������Ч��Ϊ_________��
���𰸡�������û��ܺϳ����� �� �����ĺ������� ����һӪ������ͬ�� ����������ּ��ϵ ���ҵ��� �ָ��� 2.48��1011 3.2%
��������
ͼ���У����ݼ�ͷ������ж�DΪ�����еĶ�����̼��AΪ�����ߣ�CΪ�����ߣ�BΪ�ֽ��ߣ�ͼ���У���Ϊ���ӵ������������ӵ���������ȥ�����������е������ۼ�Ϊ���ӵ�ͬ��������Ϊ�����ǵ���������Ϊ����ֽ��ߵ�������
��1�����ݷ�����֪��ͼ��DΪ�����еĶ�����̼��AΪ�����ߣ�Dָ��A�ļ�ͷ���������������ߵĹ�����û��ܺϳ����á�
��2����ͼ�ҵ�ʳ�����У����ӵķ�����ڲݵIJ��࣬�����ڲݵ�ͬ������һ���֡�
��3��ͼ�����ڵ���̬ϵͳ�У��ݹ̶�������һ�����ں������������ܵ���ʽɢʧ��һ����������������������ֳ�����߳���δ���õIJ��ֺͷֽ��߷ֽ�����֮�⣬������������һӪ������
��4����ͼ�ҵ�ʳ�����У���ʳ���뱻��ʳ��ͨ����Ϣ���ݵ����ּ��ϵ��ά����������������ȶ�����ά����̬ϵͳ���ȶ���
��5��ͼ����ʾ��̬ϵͳ������ӥ֮���õ����������ڷ����仯�����մﵽƽ�⣬�������̬ϵͳ�ڲ�����һ�������ҵ�����������ɭ����̬ϵͳ��ȣ���ԭ��̬ϵͳӪ���ṹ���ֿ����ȶ��Խ������ָ����ȶ��Խ�ǿ��
��6�������ߵ�ͬ������������̬ϵͳ��������������A��ͬ���������A���������ߣ��������̬ϵͳ����Ⱥ�����������2.48��1011 J/(hm2��a)�����������ݼ��Ĺ��ɣ�BΪ����Ӫ������CΪ�ڶ�Ӫ����������̬ϵͳ�ڶ�Ӫ����������Ӫ��������������Ч���Ƕ���ͬ�����ı�ֵ��Ϊ2.40��107/(7.50��108��= 3.2%��

����Ŀ���������
�ɺ����οɵ�����в�ȣ�в��ָ��ֲ������˺��Ļ���������ʱֲ������Na+��Ϊ�о���ԭ������Ա������ֲ������Ҷ��Ϊ���ϣ���һ��ֲ�ﲻ�����õ��л���PEG��ģ����в�ȣ�������Һ�����Ӳ�ͬŨ�ȵ�NaCl�����о����NaCl����в���µ�����Ҷ��������Ӱ�졣��ͼΪ������ù���ʾ��ͼ��A��C��ʾ���ʣ��١��ڱ�ʾ���̡���Ϊʵ��10���Ľ����
��� | ���� | ֲ��������ӣ�g�� | ��������ʣ���molm-2s-1�� | Ҷ���غ�����mgg-1�� | ҶƬK+������mgg-1�� | ҶƬNa+������mgg-1�� |
A | Ӫ��Һ | 8.1 | 23.8 | 2.43 | 24.5 | 4.1 |
B | Ӫ��Һ+PEG | 3.0 | 0 | 2.02 | 24.5 | 4.0 |
C | Ӫ��Һ+PEG+10mmolL-1NaCl | 5.3 | 8.7 | 2.58 | 23.8 | 10.9 |
D | Ӫ��Һ+PEG+20mmolL-1NaCl | 7.1 | 15.3 | 2.61 | 23.4 | 16.8 |
E | Ӫ��Һ+PEG+40mmolL-1NaCl | 6.4 | 11.3 | 2.56 | 24.0 | 18.8 |
��1��ͼ����ĸA��ʾ______��B��ʾ______��
��2��������Ҷ��������еĹ�����______����ͼ�б�ţ�������Ծ��ѧ��ת��Ϊ�ȶ���ѧ�ܵĹ�����______����ͼ�б�ţ���
��3��������Ա��Ϊ��Ϊ��ʹʵ��������˵��������Ҫ������һ������Ķ���ʵ�飬���������Ϊ��������______��
A��Ӫ��Һ+20mmolL-1NaCl
B��PEG+20mmolL-1NaCl
C��Ӫ��Һ+80mmolL-1NaCl
D��PEG+80mmolL-1NaCl
��4�����ݱ��е�ʵ��������������˵����ȷ����______����ѡ����
A��B�������PEG����10�������Ҷ�����ٽ��й������
B��C��E���У�����Ҷ������ͨ������Na+��������ǿ��ˮ����
C������Ҷ���Կ���в����ҶƬK+������ϵ����
D��������NaCl���ܻ������Ҷ����������Ӱ��
��5�����ݱ��е�ʵ������˵������Һ������NaCl����в���µ�����Ҷ���ĸ���������������������Ӱ�죿������ԭ��______��