��Ŀ����
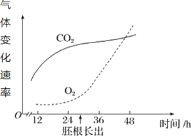
����Ŀ����÷��ʵ��ζ���أ��������У����кܸߵ�Ӫ���ͱ�����ֵ��������÷������÷�ƺ���÷��Խ��Խ��������������������������ͼ��ʾ���ش��������⣺

��1���������������У���ĸ�������ƾ��ij���Ϊ_________________���ý�Ӧ�ÿ��Ƶ��¶�Ϊ________����д�����Ʒ��͵ķ�Ӧʽ��____________________��
��2����ͼ��ʾ���Ʒ������У�����Һ���Ƕȣ������ǵ�Ũ�ȣ��;ƾ��ȣ��ƾ�Ũ�ȣ���ʱ��ı仯��ϵ������ǰ24h���Ƕȱ仯��С���ƾ���������������ԭ����___________________________________��96h��ƾ��Ⱥ��Ƕȵı仯������ƽ������ԭ����__________________________________��

��3����÷����������Ҫ�������ϵ�ͨ����������ԭ����________________����ĸ���ʹ�����ڽṹ����������������_______________________��
���𰸡�ϸ���ʻ��� 18~25�� C6H12O6![]() 2C2H5OH + 2CO2 +�������� �˽ν�ĸ����Ҫ��������������������ֳ ��Ũ�ȵľƾ��ʹ�л���������˽�ĸ���������ͷ����� ������Ǻ�����ϸ�� ��ĸ�����Ժ�ĤΪ����ϸ���ˣ���������Ժ�ĤΪ����ϸ����
2C2H5OH + 2CO2 +�������� �˽ν�ĸ����Ҫ��������������������ֳ ��Ũ�ȵľƾ��ʹ�л���������˽�ĸ���������ͷ����� ������Ǻ�����ϸ�� ��ĸ�����Ժ�ĤΪ����ϸ���ˣ���������Ժ�ĤΪ����ϸ����
��������
���Ƶ������벻����ĸ������ĸ���Ǽ���������������������½�����������������ֳ�������������½��оƾ����͡�20���������ʺϽ�ĸ����ֳ���ƾ�����ʱһ�㽫�¶ȿ�����18~25�����������һ�ֺ�����������������Դ������ʱ�����������֭�е��Ƿֽ�ɴ��ᣬ��ȱ����Դʱ����������Ҵ������ȩ���ٽ���ȩ��ɴ��ᡣ
��1����ĸ���ľƾ������������IJ�������������ϸ���ʻ��ʣ����Ʒ���������Ҫ���ƵĻ����¶�Ϊ18~25�棬�÷�Ӧ���̵ķ�Ӧ����ʽΪ: C6H12O6![]() 2C2H5OH + 2CO2 +����������
2C2H5OH + 2CO2 +����������
��2����ĸ���ڳ�����Ҫ������������������ֳ���˽����ĵ������ǽ��٣����Ҽ������������������������ľƾ�����Ҳ���٣���Σ����ھƾ�Ũ�����ߣ���л������ۣ�Ӱ���˽�ĸ���������ͷ����̵���������ľƾ����١�
��3��������Ǻ�����ϸ�����䷱ֳ��Ҫ�����������������Ҫ�������ϵ�ͨ���������ĸ�������ϸ�����������ԭ��ϸ���������ڽṹ���������������ǽ�ĸ�����Ժ�ĤΪ����ϸ���ˣ���������Ժ�ĤΪ����ϸ���ˡ�

 ���㼤�������100�ִ��Ծ�ϵ�д�
���㼤�������100�ִ��Ծ�ϵ�д�