��Ŀ����
����Ŀ����ش��������⣺
��1����ͬһֲ��ϸ�����ν�������ˮ��0.3 mol/L��������Һ��0.4 mol/L��KNO3��Һ�У����ϸ���������ʱ��ı仯������ͼ��ʾ��
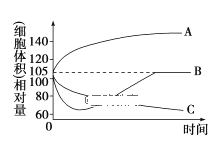
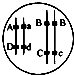
������A��B��C�ֱ����ϸ����������Һ��_________________��
�ڴﵽƽ���________��Һ��ϸ����ϸ��ҺŨ�����
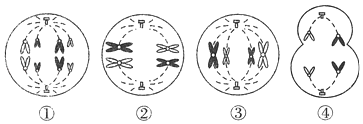
��2����ͼ��ij�������С���ڹ۲�ֲ��ϸ���ʱڷ����ԭʵ��ʱ���۲쵽�ļ�����Ƭ��������ѧ��֪ʶ��������ͼ���ش����⣺

�ٷ����ʱڷ����________����������ʹ�Ѿ��ʱڷ����ֲ��ϸ�������ʱڷ��븴ԭ��
����������ͼ����ʾ����ͬһ��֯��ϸ������ʱϸ��Һ��Ũ��������________(����ͼ�е����ֱ�ʾ)�е�ϸ����
��3�����������ͼ��ij�о�С��Χ��̽��H2O2�ֽ���������õ�ʵ�������Իش��������⣺
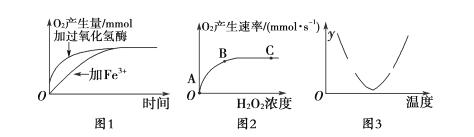
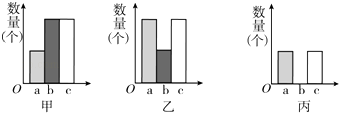
��ͼ1��2��3��������ʵ���У�ʵ���Ա�������Ϊ_____________��______________��________________��
�ڸ���ͼ1���Եó���ʵ�������___________________________��
���𰸡�����ˮ��KNO3��Һ��������Һ���Ǽ�������ˮ2��������H2O2Ũ���¶�ø�Ĵ����þ��и�Ч��
��������
���⿼�����������ʱڷ����ԭʵ�顢ø���ص�����ʵ���������ڿ��鿼����������ѧ֪ʶ��ͨ���Ƚϡ��������ۺϵȷ�����ͼ�����н��͡������������������жϻ�ó���ȷ�Ľ���������
��1���پ����߷�����A����ϸ��������ӵ�һ���̶Ȳ������ӣ��Ǵ�����ˮ�е�ϸ����ֲ��ϸ���������ͣ���һ��ʱ���ˮ�ֳ���ƽ�⡣
B����ϸ����ʧˮ������ˮ��Ϊ����0.4mol/L��KNO3��Һ�У�һ��ʼ��ϸ��ʧˮ��һ��ʱ�����������ͨ���������䲻�Ͻ���Һ�ݣ�����ϸ��ҺŨ�ȣ�������ˮ��
C���ߴ���ϸ��λ��0.3mol/L��������Һ��ϸ��ʧˮ�����С����һ��ʱ���ϸ��ҺŨ�Ⱥ������ҺŨ���൱��ˮ�ֳ���ƽ�⣬ϸ����������нϴ�仯��
������A��B��C�ֱ����ϸ����������Һ������ˮ��KNO3��Һ��������Һ��
��ʧˮԽ�࣬�ﵽƽ���ϸ����ϸ��ҺŨ�����������������Һ��
��2���ٷ����ʱڷ��������ˮ����������ʹ�Ѿ��ʱڷ����ֲ��ϸ�������ʱڷ��븴ԭ��
���ʱڷ���̶�Խ��ʧˮԽ�࣬ϸ��Һ��Ũ��Խ��������2�е�ϸ����
��3����ͼ1��2��3��������ʵ���У�ʵ���Ա�������Ϊ�������ࡢH2O2Ũ�ȡ��¶ȡ�
�ڸ���ͼ1��֪�������������ø����ͬʱ��������������࣬���ʵ�������ø�Ĵ����þ��и�Ч�ԡ�