��Ŀ����
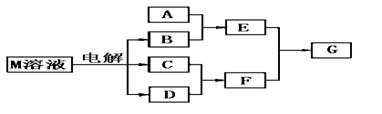
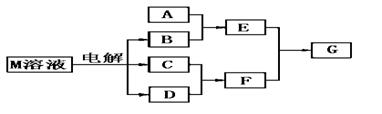
��֪����M������ͬһ���ڵ�X��Y���ֶ�����Ԫ����ɣ�Xԭ�ӵ����������������ڲ��������һ�룬YԪ��������������ĸ��۴�����Ϊ6��M���������ʵ�ת����ϵ���£����ֲ�������ȥ����

��1����֪Ԫ��Z��Y��������������ͬ�Ķ�����Ԫ�أ���ôZ��Y�ֱ������γɵ��⻯���зе�ϸߵ��� ���ѧʽ����ԭ���� ��
��2����A��һ�ֳ���������������ҿ��������첣������E��Һ��F��Һ��Ӧ�����ӷ���ʽ�ǣ�
��
��3����A����X��Yͬ����Ԫ�ص�һ�ֳ����������ʣ���A��B��Һ�ܹ���Ӧ�����仯ѧ����ʽ�ǣ�
��
��4����A��һ�ֳ����Ļ��ʣ�ʽ��Ϊ79����E��F����Gʱ�а��̲�������G�ĵ���ʽ�ǣ�
 ��
��
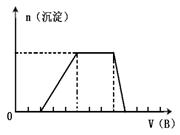
��5����A��һ����Һ��ֻ���ܺ���H+��NH4+��Mg2+��Fe3+��Al3+��CO32����
SO42���е�ijЩ���ӣ��������Һ�м���B��Һʱ�������ɳ��������ʵ�����B��Һ������仯��ͼ��ʾ���ɴ˿�֪������Һ�п϶����е������� ��������Ħ��������С������ͬ��,���ʵ���Ũ��֮��Ϊ ��
��1��HF(1��)��HF����֮������γ����(1��)��
��2��SiO32-+2H++2H2O= H4SiO4����SiO32-+2H+= H2SiO3��(2��)��
��3��2NaOH+2H2O+2Al=2NaAlO2+ 3H2��(2��)��
��4��![]() (2��)��
(2��)��
��5��H+��NH4+��Al3+��SO42-(3��)��2��3��1��4(3��)��
����:
��

 ��
��
 ��
��