��Ŀ����
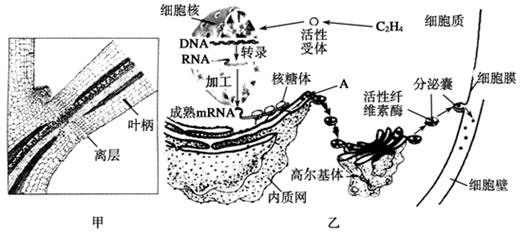
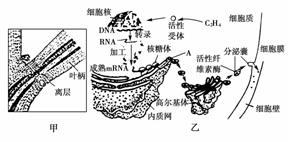
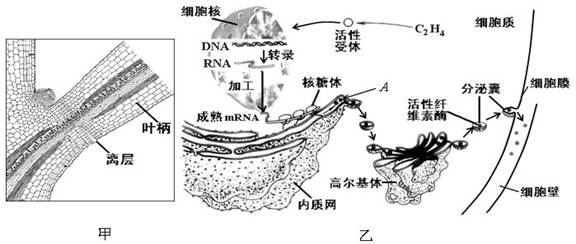
��ĩֲ���ҶƬ˥��ʱ����Ҷ��������ʼ�γ���㣨��ͼ����ʾ���������ֲ���������䡣��㲿��ϸ����ص��������ͼ����ʾ�����ͼ�ش��������⣺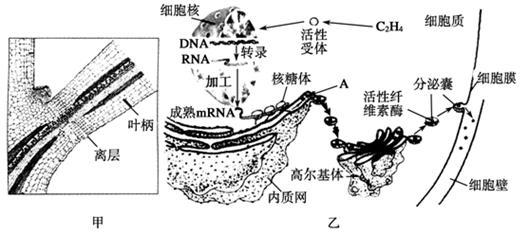
��1����ͼ�ҿ�֪��Ҷ���������IJ������� ��ֲ�D�أ������й�ϵ���ڸ�ֲ�D�ص������£�ϸ�����е��йػ�����б������ı�����̵ij����ֱ��� �� ��
��2��A�������������Գ�ѿ�ķ�ʽ�γ�С�ݣ������߶����壬���ɸ߶������γɷ����ң�����ϸ��Ĥ������ͨ��ϸ���� ���ã����ڵ�ϸ���⡣��һϵ�еĹ���֤�� �� ��
��3����һ�����о�������Ҷ�����������γɣ����������������ֲ�D�صIJ��룬��˵�� ��
��4��˥��ҶƬ��N��P��������Ҷ�к��� ��
��1����ϩ ϸ���� ������
��2������ ����Ĥ����һ���������� ��������Ĥ�ڽṹ�����Ͼ���һ����������
��3��ֲ���������ڣ����ǵ�һ���ص����ã������ɶ��ּ��ع�ͬ���ڵģ�4�����١�
����

 ��Ҷ��������ʼ�γ���㣨��ͼ����ʾ���������ֲ���������䡣��㲿λϸ����ص��������ͼ����ʾ�����ͼ�ش�����
��Ҷ��������ʼ�γ���㣨��ͼ����ʾ���������ֲ���������䡣��㲿λϸ����ص��������ͼ����ʾ�����ͼ�ش����� ���⣺
���⣺ ��1������ͼ��֪��Ҷ���������IJ�������ߣߣߣߣ�ֲ�D�أ������й�ϵ��
��1������ͼ��֪��Ҷ���������IJ�������ߣߣߣߣ�ֲ�D�أ������й�ϵ�� 2��A�������������Գ�ѿ�ķ�ʽ�γ�С�ݣ������߶����壬��
2��A�������������Գ�ѿ�ķ�ʽ�γ�С�ݣ������߶����壬�� �ɸ߶������γɷ����ң�����ϸ��Ĥ������ͨ��ϸ��Ĥ�ģߣߣߣ����ã����ڵ�ϸ���⡣
�ɸ߶������γɷ����ң�����ϸ��Ĥ������ͨ��ϸ��Ĥ�ģߣߣߣ����ã����ڵ�ϸ���⡣ ���ֶβ��������������Ϊ������������ֲ����澳�ĵֿ�������ʹ�����в������һ��ʵ�飬��̽���������ֲ����Ե�Ӱ�졣������Ʒ������������ƿ��״̬������һ�µĻƹ�����10�ꡢ����Ũ�ȵ������ᡢ�ⶨ�絼�ʵ�װ�á�0.25mol��L NaCl��Һ������ˮ��
���ֶβ��������������Ϊ������������ֲ����澳�ĵֿ�������ʹ�����в������һ��ʵ�飬��̽���������ֲ����Ե�Ӱ�졣������Ʒ������������ƿ��״̬������һ�µĻƹ�����10�ꡢ����Ũ�ȵ������ᡢ�ⶨ�絼�ʵ�װ�á�0.25mol��L NaCl��Һ������ˮ�� �ڶ������ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣ�
�ڶ������ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣ�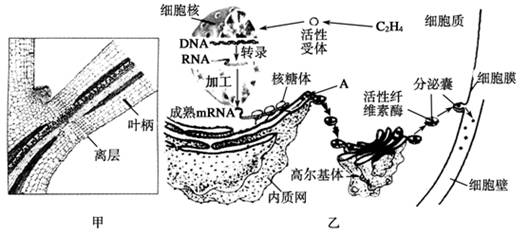
 ��1������ͼ��֪��Ҷ���������IJ�������ߣߣߣߣ�ֲ�D�أ������й�ϵ��
��1������ͼ��֪��Ҷ���������IJ�������ߣߣߣߣ�ֲ�D�أ������й�ϵ��