��Ŀ����
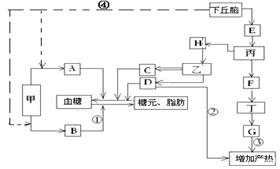
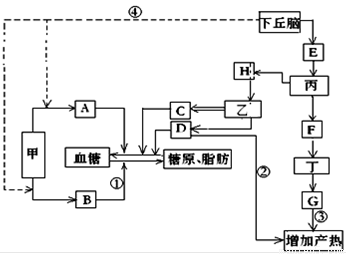
��ͼΪ�����Զ����岿����������ڵ�ʾ��ͼ��ͼ��Ӣ����ĸ��ʾ���أ��ס��ҡ���������ʾ�ڷ����٣����ֱ�ʾ��������������У�����C�ķ�����H�ĵ���Ӱ�졣���߲��ֱ�ʾ������1������B�������ǣߣߣߣߣ�ͼ���뼤��AЭͬ��������Ѫ��Ũ�ȵļ����� [ ] �ߣߣߣߺ�[ ] �ߣߣߣߡ�����������Ӣ����ĸ��������д�������ƣ�
��2����ͼ�еĵ��ع�ϵ�жϣ�E�����ǣߣߣߣߣߣߣߣߣߣߣߣߣ��������ǣߣߣߣߡ�
��3������ҽ���������������������������������ѧ֪ʶ˵��ҽ�������ݣ��ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߡ�
��4��F����ֻ����Ϊ�����źţ��붡ϸ��Ĥ�Ͼ����ض��ģߣߣߣߣ����ʣ��йء�����̼���D���ʶ����µ��ڵľ���������Ҫ�ǣߣߣߣߡ�
��5�����ܵ�����̼�ʱ������ͼ����ʾ�����������������ı��⣬����������ʽ�������µ�ЧӦ���Уߣߣߣߡ�
��1�� �ȵ��� C��D ������Ƥ�ʼ��غ���������
��2���ټ�״�ټ����ͷ��غʹ�������Ƥ�ʼ����ͷ��� ����
��3�������˵�Ѫ��Ũ�ȵĻ���ˮƽ�Ѿ��ϸߣ�����������ʱ���������ط������ӣ�Ѫ��Ũ�����ߡ�ͬʱ���������������˷ܣ�����ԭ�ֽ�ӿ죬��һ�����Ѫ��Ũ�ȣ��к�����
��4���ǵ��� �������ڴ�л���ǿ�����������ӣ�5��Ƥ��Ѫ�ܡ���ë�������١�������
��������

 Ŀ�����ϵ�д�
Ŀ�����ϵ�д�

 ATP������Ӧ��
ATP������Ӧ��