��Ŀ����
��4�֣���Ȼ���е������ǻ��������ġ��������о�����ʱһ��Ҫ�����Ƿ����ᴿ��Ȼ����������IJⶨ�������ǶԴ˾����������ⶨ��ʵ�飬��ش��й����⡣
���裺���Ʊ�ϡ�ͱ���Ϊ102��103��104��105��106��ϵ��ϡ��Һ��
����ϡ��Ϳ��ƽ�巨������Ʒ��
�������¶���������
���������
��1���ⶨ�Ĵ˾������ڶ�Ӧϡ�ͱ���Ϊ106���������еõ����¼���ͳ�ƽ������ȷ���ŵ��� ��
A��һ��ƽ�壬ͳ�Ƶľ�������23
B������ƽ�壬ͳ�Ƶľ�������22��26��ȡƽ��ֵ24
C������ƽ�壬ͳ�Ƶľ������ֱ���21��5��52��ȡƽ��ֵ26
D���ĸ�ƽ�壬ͳ�Ƶľ������ֱ���21��30��24��25��ȡƽ��ֵ25
��2��һͬѧ��ϡ�ͱ���Ϊ106���������в��ƽ���Ͼ�������ƽ��ֵΪ23.4����ôÿ������Ʒ�еľ������ǣ�Ϳ��ƽ��ʱ����ϡ��Һ�����Ϊ0.2ml�� .
(3)�����ַ����ⶨ�ܶȣ�ʵ�ʻ������Ҫ�Ȳ�õ����� ��
��4��ijͬѧ�ڲⶨ�˾����ܶ�ʱ���֣����������ϻ��������Ӿ��ľ��䣬�Ⲣ���ܿ϶��ô˾���Ʒϵ����Ⱦ�ˣ���Ϊ��
��
��7�֣���ͼ����ɫֲ��Ҷ��ϸ���й�������������������̼����ϵ��ͼ�⣬����A-D��ʾ��ع��̣�a-e��ʾ�й����ʣ���ͼ�ش�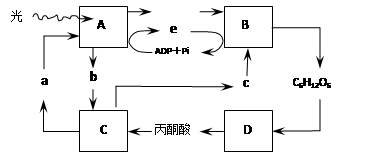
|
��3�����������ϴ��������߲�ʱ�������������������������ɲ�ȡ�ʵ�����_____________�Ĵ�ʩ������___________������ĸ�����̣��������л���Ļ��ۡ�
��4����ͻȻֹͣ���գ����������C3�ĺ����仯�� ��
��1��D ��2��23.4��0.2*106=1.17*108(��)
��3���� ��4�������ų��Ӿ������������������������̡�
��1���ⷴӦ O2 ��2��Ҷ������� ������
��3���¶� C��D ��5������
����

 ����������ϵ�д�
����������ϵ�д� �Ż���ҵ�Ϻ��Ƽ����׳�����ϵ�д�
�Ż���ҵ�Ϻ��Ƽ����׳�����ϵ�д�������̬ϵͳ��������������˵��������ǣ�������
| A����ʳ�����У����������ǵ���ģ���Ϊ��һ��Ӫ���������ﲻ��������һ��Ӫ����������Ϊʳ |
| B�������ݼ�������ԭ��֮һ�Ǻ������� |
| C�������������ص�˵����Ȼ���е������Dz��غ�� |
| D�������Ĵ���Ч�ʴ�ԼΪ10����20�� |
��4�֣���Ȼ���е������ǻ��������ġ��������о�����ʱһ��Ҫ�����Ƿ����ᴿ��Ȼ����������IJⶨ�������ǶԴ˾����������ⶨ��ʵ�飬��ش��й����⡣
���裺���Ʊ�ϡ�ͱ���Ϊ102��103��104��105��106��ϵ��ϡ��Һ��
����ϡ��Ϳ��ƽ�巨������Ʒ��
�������¶���������
���������
��1���ⶨ�Ĵ˾������ڶ�Ӧϡ�ͱ���Ϊ106���������еõ����¼���ͳ�ƽ������ȷ���ŵ��� ��
A��һ��ƽ�壬ͳ�Ƶľ�������23
B������ƽ�壬ͳ�Ƶľ�������22��26��ȡƽ��ֵ24
C������ƽ�壬ͳ�Ƶľ������ֱ���21��5��52��ȡƽ��ֵ26
D���ĸ�ƽ�壬ͳ�Ƶľ������ֱ���21��30��24��25��ȡƽ��ֵ25
��2��һͬѧ��ϡ�ͱ���Ϊ106���������в��ƽ���Ͼ�������ƽ��ֵΪ23.4����ôÿ������Ʒ�еľ������ǣ�Ϳ��ƽ��ʱ����ϡ��Һ�����Ϊ0.2ml�� .
(3)�����ַ����ⶨ�ܶȣ�ʵ�ʻ������Ҫ�Ȳ�õ����� ��
��4��ijͬѧ�ڲⶨ�˾����ܶ�ʱ���֣����������ϻ��������Ӿ��ľ��䣬�Ⲣ���ܿ϶��ô˾���Ʒϵ����Ⱦ�ˣ���Ϊ��
��
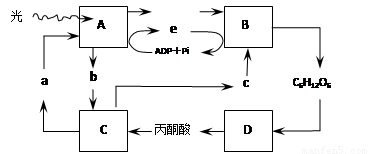
��7�֣���ͼ����ɫֲ��Ҷ��ϸ���й�������������������̼����ϵ��ͼ�⣬����A-D��ʾ��ع��̣�a-e��ʾ�й����ʣ���ͼ�ش�
|
��2��B��C���̽��еij����ֱ���______________��_______________��
��3�����������ϴ��������߲�ʱ�������������������������ɲ�ȡ�ʵ�����_____________�Ĵ�ʩ������___________������ĸ�����̣��������л���Ļ��ۡ�
��4����ͻȻֹͣ���գ����������C3�ĺ����仯�� ��