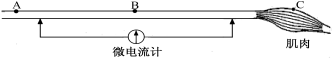
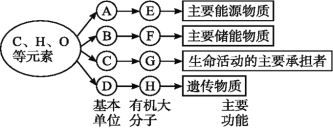
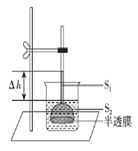
��Ŀ����
����Ŀ�����������ǵ����������������ڣ����\�������ǵ���������˺ܴ�ĸı䣬��ش���������
��1���ƹ���ʱ��Ӧ�õľ���Ϊ_____________���ƹ���ʱӦ�õľ���Ϊ_____________������֮�����������������ں���____________________________________��
��2���ƾ�ʱ��Ӧ�ȳ�ϴ���ѣ���ȥ֦������ϴ����ҪĿ����__________________��
��3�������������ѾƳ����ɫ��������ɫ������Դ��_______________��
��4�����������Ƿ�ɹ����跢�ͺ���_____________�������������������£���������ƾ���Ӧ����________ɫ��
��5����������Ѿ��������Ѵף���д����Ӧʽ��_____________________________________��
���𰸡���ĸ�� ����� ���Ժ�ĤΪ����ϸ���� ϴȥ���� ����Ƥ�е�ɫ�� �ظ���� ���� C2H5OH+ O2�� CH3COOH+ H2O
��������
1��������������������ǽ�ĸ�������³´�л����Ϊ�������������ͣ�����������ԭ����
��1�������������£���Ӧʽ���£�![]() ����2�������������£���Ӧʽ���£�
����2�������������£���Ӧʽ���£�![]() ��
��
2��������������������Ǵ���������³´�л���������������ͣ�����������ԭ��������������Դ������ʱ�������������֭�еĹ��Ƿֽ�ɴ��ᣮ��ȱ����Դʱ����������Ҵ���Ϊ��ȩ���ٽ���ȩ��Ϊ���ᣮ
��1���ƹ���ʱ��Ӧ�õľ���Ϊ��ĸ�����ƹ���ʱӦ�õľ���Ϊ���������ĸ���������������������ԭ���������֮�����������������ں������Ժ�ĤΪ����ϸ���ˣ�
��2���ƾ�ʱ��Ӧ�ȳ�ϴ���ѣ���ȥ֦������ϴ����ҪĿ����ϴȥ������
��3�����ѾƵ���ɫ��������ƤҺ���е�ɫ�أ�
��4�����������£��ظ������ƾ���Ӧ���ֻ���ɫ��
��5�������Ѿ��������Ѵķ�ӦʽΪ��C2H5OH+ O2�� CH3COOH+ H2O��