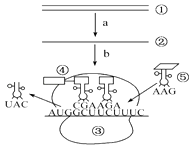
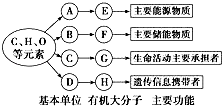
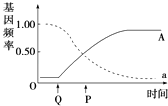
��Ŀ����
����Ŀ���������֪ʶ���ش���ڹ��ơ�������������������⣮
��1������������Դ������ʱ��������֭�е��Ƿֽ�ɴ��ᣮ
��2����ͳ�����������Ѿ�ʱ����ĸ������Դ�� �� ��ĸ�����������ķ�ӦʽΪ �� �������£��ظ������ƾ�������Ӧ����ɫ��
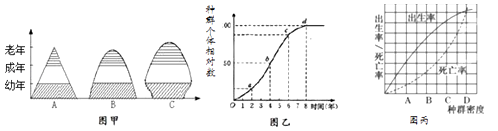
��3����ͼΪ����������ʵ������ʾ��ͼ����ͼ�ش��������⣮ ��ͼ��A�������Ǽ����ƣ�����ҪĿ�������������е�ˮ�֣�ʹ�������Ӳ��ͬʱ���� ��
��������±���оƵĺ���һ�������12%���ң��Ӿƺ������߸������ʱ�佫�����ƾ��������ͣ����������������������ܵ��¶������ܣ�
���𰸡�
��1�������
��2�����ѣ� C6H12O6![]() 2CO2+2C2H5OH+���������ԣ�����ɫ
2CO2+2C2H5OH+���������ԣ�����ɫ
��3���Σ��������������,���ⶹ���鸯�ܱ��ʣ��ӳ�������
���������⣺��1������������Դ������ʱ�������������֭�еĹ��Ƿֽ�ɴ����ȱ����Դʱ����������Ҵ���Ϊ��ȩ���ٽ���ȩ��Ϊ���ᣮ��2����ͳ�����������Ѿ�ʱ����ĸ���������ѣ��Ǹ���������Ƥ�ϵ�Ұ���ͽ�ĸ������ĸ�����������ķ�ӦʽΪC6H12O6 ![]() 2CO2+2C2H5OH+�������ƾ������������ظ������Һ��������Һ����ɫ�ɳ�ɫ��ɻ���ɫ����3���ٸ�������������Ϊ��
2CO2+2C2H5OH+�������ƾ������������ظ������Һ��������Һ����ɫ�ɳ�ɫ��ɻ���ɫ����3���ٸ�������������Ϊ�� ![]() �����ͼ��A�������Ǽ������ƣ�����ҪĿ�������������е�ˮ�֣�ʹ�������Ӳ��ͬʱ����������������������ⶹ���鸯�ܱ��ʣ�
�����ͼ��A�������Ǽ������ƣ�����ҪĿ�������������е�ˮ�֣�ʹ�������Ӳ��ͬʱ����������������������ⶹ���鸯�ܱ��ʣ�
��������±���оƵĺ���һ�������12%���ң��Ӿƺ������߸������ʱ�佫���ӳ����ƾ��������ͣ�������������������������ܵ��¶������ܣ�
���Դ��ǣ���1���������2������ C6H12O6 ![]() 2CO2+2C2H5OH+���� ���� ������ɫ ��3�����Ρ���������������������ⶹ���鸯�ܱ��ʡ����ӳ� ����
2CO2+2C2H5OH+���� ���� ������ɫ ��3�����Ρ���������������������ⶹ���鸯�ܱ��ʡ����ӳ� ����

����Ŀ����֪�㶹��������Ҷ�Ļ�ɫ����ɫ��һ�Ե�λ����Y��y���ƣ������㶹���������Ŵ�ʵ�飬������ش�
ʵ��һ | ʵ��� |
P��ɫ��Ҷ�ס���ɫ��Ҷ�� | P��ɫ��Ҷ�� |
��1�����㶹���Ŵ�ʵ������ȡ�óɹ���ԭ��֮һ�� ��
��2����ʵ�����ж���������״����������״��
��3��ʵ��һ�Ӵ��г��ֻ�ɫ��Ҷ����ɫ��Ҷ�ı���Ϊ1��1������Ҫԭ���ǻ�ɫ��Ҷ�ײ������������༰�����Ϊ ��
��4��ʵ�����ɫ��Ҷ��Ļ�����Ϊ �� �������ȶ��Ŵ���ռ �� ����ɫ��Ҷ��ֲ��֮��������䣬����õ��Ӵ�����ɫ��Ҷռ ��
��5��ʵ��һ�л�ɫ��Ҷ����ʵ����л�ɫ��Ҷ���ӽ�������õ��Ӵ���ɫ��Ҷ�����в����ȶ��Ŵ���ռ ��