��Ŀ����
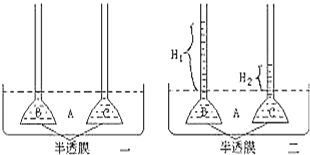
����Ŀ��ͼ��һ������ƽ��״̬����̬ϵͳ����������ͼ�⣬����A��B��C��D�ֱ������ͬӪ������������Ⱥ���Դ�ͼ������ȷ���ǣ� �� 
A.��������̬ϵͳ����������A�̶���̫���ܼ�ȥ�����������ĵ�����
B.�ڴ���̬ϵͳ�У���һֻ�Dz�ʳ��һֻҰ�ã�����ǻ�ø�Ұ��Լ10%��20%������
C.Bͬ��������Ҫ����B��C��D�������ĵ�����֮��
D.����������̬ϵͳ����������һ���ģ�����B��õ�����Խ�࣬����C��D��������Խ��
���𰸡�C
���������⣺A����������̬ϵͳ����������������A���̶���̫����������A���� B�������Ĵ���Ч����ָ������Ӫ����Ⱥ����ͬ����֮�ȣ�����һӪ���������������ͬ��������һӪ�������������ͬ����֮�ȣ�B����
C��Bͬ��������������ȥ�����������C�ʹ����ֽ��ߣ�������̬ϵͳ����ƽ��״̬�����������Ͽ�Bͬ������������BCD�ĺ����������ߴ����ֽ��ߵ�����������B��ͬ�����������ߺ������ĵ�������C��ȷ��
D����B��õ�����Խ�࣬���մ���Ч��10%��20%���㣬C��D��õ�������Խ϶࣬D����
��ѡ��C��
���������ͼʾ������֪����������̬ϵͳ�����������������߹̶���̫���������⣬�����˹�����������������Ĵ���Ч����ָ����Ӫ����֮��ͬ����֮�ȣ����Ǹ���֮�����Ⱥ��֮�䣻�μ���������ָ������������������ͬ������������ȥ���������������ĵ�����֮�ͣ���B��õ�����Խ�࣬���մ���Ч��10%��20%���㣬C��D��õ�������Խ϶࣮

 ������ÿ�ʱ��ҵϵ�д�
������ÿ�ʱ��ҵϵ�д�