��Ŀ����
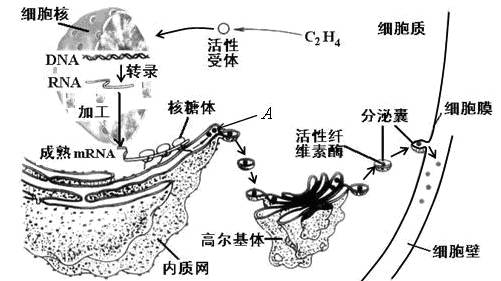
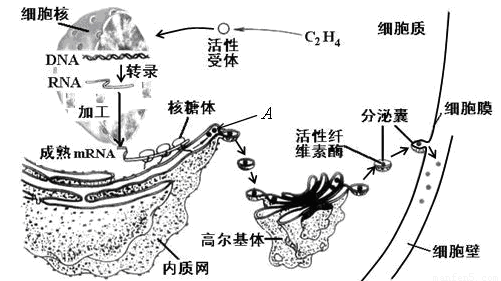
��ĩ��Ҷʱ��ֲ��Ҷ��������ʼ�γ���㣨��ͼ����ʾ��������ҶƬ����㲿λ���䡣��ͼ��ʾ��㲿λϸ����ص�����������ͼ�ش��������⣺
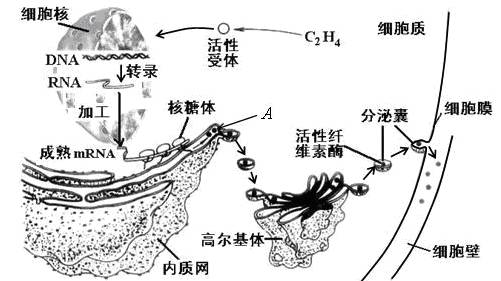
��1����ͼʾ��֪������γ�ʱ����ϩ����ϸ�����ض��������ϣ�����ϸ�����ڵ�ת¼���ӣ��ϳ�����A������A�ϳɹ������Ŵ���Ϣ��������Ϊ��
�������ֺͼ�ͷ��ʾ����
��2��A�������������Գ�ѿ�ķ�ʽ�γ�С�ݣ������߶����壬���ɸ߶������γɷ����ң�����ϸ��Ĥ������ͨ�� ���ã����ڵ�ϸ���⡣��һ����������A���ʴ�������Ĥ �㡣�߶������A���ʵ�������
��
��3���ù��̷��ڵ�ϸ��Ĥ������ʣ�ͨ�� ���ٽ�����γɡ�
��4��ֲ��ϸ��Ĥ��ά��ϸ���������������Ĵ�л������Ҫ�����á���ֲ�����澳������¡��ɺ������գ���ʱ��ϸ��Ĥ��ƻ���Ĥ�����Ӷ�ʹϸ���ڵĵ��������������ֲ��ϸ������Һ�ĵ絼������Ĥ������ij̶���ֲ����Ե�ǿ���йء���ѧ���ϱ������������ܸı�Ĥ���ԡ����Է�������Ϊʵ����Ͻ�����в�����Ʒ���ʵ�飬��̽���������ֲ����Ե�Ӱ�졣
������Ʒ������Ũ�ȵ���������Һ��0.25mol��LNaCl��Һ������ˮ���ⶨ�絼�ʵ�װ�á������������
��ʵ����裺___________________________________��
��ʵ�鲽�裺
��һ����____________________________ ____ _��
�ڶ�����_________________________ ________��
���������������ʩ ����������ʩ ��
���IJ���_________________ ________________��
��Ԥ�ڽ�������ۣ�________________________________________________�����ٴ�����㣩
��1��DNAת¼��mRNA ���������A
��2������ 0 �ӹ������䡢Ũ����ת��
��3���ֽ�ϸ����
��4��������������ֲ��Ŀ�����
ȡ���������ɣ�ƽ����Ϊ�ס������飬��ʢ�ŵ���������ˮ��
ȡ����״������С������һ�µķ����������ɷֳ����飬�ֱ����ڱ��Ϊ�ס��ҵ��������з�����ͬ��������������
һ������0.25mol/L��NaCl��Һ����������������Һ��
�òⶨ�絼�ʵ�װ�÷ֱ��ס�������ĵ絼�ʲ�����¼��
������絼�ʴ������飬��˵��������������ֲ��Ŀ����ԣ����ס�������ĵ絼��������˵�������������ֲ��Ŀ����ԣ�������絼�ʴ��ڼ��飬˵��������������ֲ����澳��
����:
��ֲ�������Ϊ�������ۺϿ������ı�����ڵ��ĺϳɼ�ʵ�����������
������A�ϳɵĹ��̾���ת¼�ͷ���Ĺ��̣������ķ����һ�����Ŵ���Ϣ����ͼ�⡣
��A���ʺϳɹ��������γ�С�ݼ������ң�������������µ���ʽ���ڵģ�������Ĥ�ںϵ���ʽ��ɣ������ڿ�Ĥ���䣬�������κ�Ĥ����A���ʺϳɷ��ڵĹ����У��߶������ӹ������䡢Ũ����ת�˵����á�
�Ǵ�ͼ�п��Կ�����������������ϸ���ڣ�Ӧ����ͨ���߽�ϸ���ڻ������������á�
��ʵ����ƹؼ�Ҫѡʵ����Ա��������������ʵ����з�����