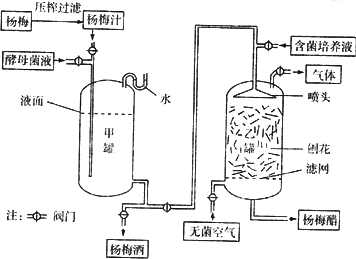
��Ŀ����
��÷������ˮ��֮һ��Ϊ���������ӹ���ij����������÷�ƺ���÷�����ƣ����������������£�

��ش�
(1)��������м�ˮ����ҪĿ����__________������һ��ʱ��۲쵽������Һ�治����_________��˵�����ͻ�����ϡ�
(2)���Ʊ���÷�����У��ҹ�������侭________������ľ���ٻ���Ȼ����뺬_________��������Һ��ʹ�þ�_________���ٻ��ϣ����ü��з�����ϵ���÷�������ҹ�����÷���ͣ���÷��pH��ͨ��������÷�Ƶ�________�����ڡ�
(3)�����е���÷��ȫ�������ҹ��Ƴ���÷�ף����ҹ���CO2�IJ�������________��
A�������� B�������� C����һ�� D������Ϊ��
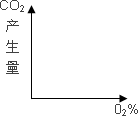
(4)����÷�ƺ���÷���͵����������У�ij����Ũ����ʱ��仯��ʾ��ͼ���£���������_________��
(1)��������м�ˮ����ҪĿ����__________������һ��ʱ��۲쵽������Һ�治����_________��˵�����ͻ�����ϡ�
(2)���Ʊ���÷�����У��ҹ�������侭________������ľ���ٻ���Ȼ����뺬_________��������Һ��ʹ�þ�_________���ٻ��ϣ����ü��з�����ϵ���÷�������ҹ�����÷���ͣ���÷��pH��ͨ��������÷�Ƶ�________�����ڡ�
(3)�����е���÷��ȫ�������ҹ��Ƴ���÷�ף����ҹ���CO2�IJ�������________��
A�������� B�������� C����һ�� D������Ϊ��
(4)����÷�ƺ���÷���͵����������У�ij����Ũ����ʱ��仯��ʾ��ͼ���£���������_________��

(5)��ĸ�����оƾ����ͷ�Ӧʽ______________��
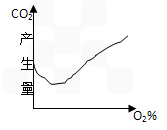
(6)����ͼ�л�����ĸ����л����CO2������O2Ũ�ȵ�����ͼ
(6)����ͼ�л�����ĸ����л����CO2������O2Ũ�ȵ�����ͼ

(1)��ֹ�������룻����ð��
(2)�����������ţ�����
(3)D
(4)�ƾ�
(5)C6H12O6 2C2H5OH+2CO2+����
2C2H5OH+2CO2+����
(6)
(2)�����������ţ�����
(3)D
(4)�ƾ�
(5)C6H12O6
 2C2H5OH+2CO2+����
2C2H5OH+2CO2+����(6)


��ϰ��ϵ�д�
�����Ŀ