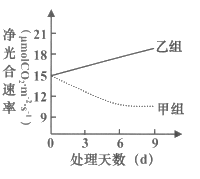
��Ŀ����
����Ŀ��ij�о�С����������Ϊԭ���������ѾƵĻ������̺�װ��ʾ��ͼ���¡���ش��������⣺

��1���ø�ѹ��������������ѽ����������������������ڸ������ܵ�������__________________��
��2���̶�����ĸϸ�����õİ������_________________�������Һ���뵽����Ũ�ȵ�CaCl2����Һ�У�CaCl2��������___________________��
��3����װ��ͼ��������С��ʵ����ڵIJ�����_______�����������________________________��
��4������⣬����ǰ�ķ���Һ�д��ڽ�ĸ��������ܵ�ԭ����_____________________��ʹ��Ѫ������壨16��25�� 1mm��lmm���ⶨ��Ũ�ȣ�ij��С�����н�ĸ���ֲ���ͼ����ͳ�Ƹ�С����Ľ�ĸ������Ϊ______________________����
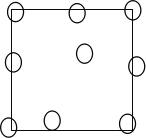
��5��ʵ���У��жϷ�����ϵ������� _____________________��
���𰸡�����ʱ�ŵ����ڵ������ �������� ʹ�������γ��ȶ��ṹ ����ķ���Һ�����ѽ�����̫�� ���ھ��己ֳ���ĵ�����̫�ࣨ�ƾ����������٣� �����Ũ��̫�ͣ��������϶̫��ĸ��©�� 6 ˮ�в���������ð��
��������
1��������������������ǽ�ĸ�������³´�л����Ϊ�������������͡�����������ԭ����
��1�������������£���Ӧʽ���£�C6H12O6+6H2O+6O2![]() 6CO2+12H2O+������
6CO2+12H2O+������
��2�������������£���Ӧʽ���£�C6H12O6![]() 2CO2+2C2H5OH+������
2CO2+2C2H5OH+������
2�����ð����Ʊ��̶���ĸϸ������Ҫ���裺��ĸϸ���Ļ����������ˮʹ�ɽ�ĸ�ָ���������״̬�����������ơ�CaCl2��Һ�����ƣ����ܽ⺣��������Һ�Ĺ�����ʹ��С����ϼ��ȣ���ֹ��Һ������������������Һ���ĸϸ����ϣ�ע����ȴ����������Һ�����£���ֹ�����ƻ���ĸ���Ļ��ԣ����̶�����ĸϸ����
��1��������������ڸ������ܵ������Ƿ���ʱ�ŵ����ڵ��������
��2���̶�����ĸϸ�����õİ�����Ǻ������ƣ������Һ���뵽����Ũ�ȵ�CaCl2����Һ�У�CaCl2��������ʹ�������γ��ȶ��ṹ��
��3����װ��ͼ��������С��ʵ����ڵIJ����Ǽ���ķ���Һ�����ѽ�����̫�٣�������������ھ��己ֳ���ĵ���̫����ƾ����������١�
��4������⣬����ǰ�ķ���Һ�д��ڽ�ĸ��������ܵ�ԭ���ǰ����Ũ��̫�ͣ��������϶̫��ĸ��©����ʹ��Ѫ������������ĸ������������ԭ���DZ߿���ϲ����£��������ټ������еĸ�����ͨ��ͼʾ��������6����
��5����ĸ��ϸ����������������̼����ʵ���У��ɸ���ˮ�в���������ð��
�жϷ�����ϡ�

 ���Ǽ���С����ϵ�д�
���Ǽ���С����ϵ�д�