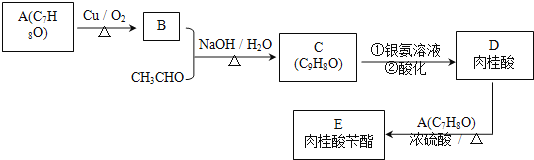
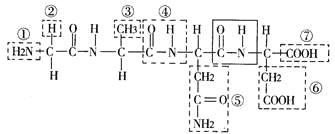
جâؤ؟ؤعبف
،¾جâؤ؟،؟I،¢µبخïضتµؤء؟µؤCH4؛حC3H8حêب«ب¼ةصت±£¬؛ؤرُء؟ا°صك________؛َصك£»µبضتء؟µؤCH4؛حC3H8 حêب«ب¼ةصت±£¬؛ؤرُء؟ا°صك________؛َصك£»بô؛ؤرُء؟دàح¬£¬شٍ¶صكµؤخïضتµؤء؟ض®±بn(CH4)£؛n(C3H8)£½________،£
II،¢بçح¼ؤ³ئّجهX؟ةؤـسةH2،¢CO،¢CH4ضذµؤز»ضض»ٍ¼¸ضض×é³ة£¬½«Xئّجه ب¼ةص£¬°رب¼ةص؛َةْ³ةµؤئّجهح¨¹A،¢Bء½¸ِد´ئّئ؟،£تش»ط´ًدآءذختجâ،£
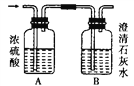
(1)بôAد´ئّئ؟µؤضتء؟شِ¼س£¬Bد´ئّئ؟µؤضتء؟²»±ن£¬شٍئّجهXتا________،£
(3)بôA،¢Bء½¸ِد´ئّئ؟µؤضتء؟¶¼شِ¼س£¬شٍئّجهX؟ةؤـتا________،£
III،¢ (1)C5H12Oµؤ´¼,شعز»¶¨جُ¼دآؤـ·¢ةْ´ك»¯رُ»¯·´س¦ةْ³ةب© ,´¼µؤµبذ§اâسذخهضض,ذ´³ِآْ×مةدتِجُ¼µؤ´¼µؤ½ل¹¹¼ٍت½£؛______________،£
(2)C5H12Oµؤ´¼,شعز»¶¨جُ¼دآ²»ؤـ·¢ةْدûب¥·´س¦,ذ´³ِآْ×مجُ¼µؤ´¼µؤ½ل¹¹¼ٍت½£؛___________،£
(3)·ض×ست½خھC5H12O,²»ؤـسë½ًتôؤئ·´س¦،£µبذ§اâسذثؤضض,µبذ§اâµؤ¸ِت±بخھ3،أ2،أ1،أ6,ذ´³ِآْ×مةدتِجُ¼µؤخïضتµؤ½ل¹¹¼ٍت½______________________________________________،£
،¾´ً°¸،؟ ذ،سع ´َسع 5£؛2 H2 CH4»ٍH2،¢CO»ٍH2،¢CH4»ٍCO،¢CH4»ٍH2،¢CO،¢CH4 £¨CH3£©2CHCH2CH2OH £¨CH3£©3CCH2OH £¨CH3£©2CHCH2OCH3£»£¨CH3£©2CHOCH2CH3
،¾½âخِ،؟I،¢ح¬ضتء؟µؤجCxHy£¬ ![]() ضµش½´َ£¬حêب«ب¼ةص؛ؤرُء؟ش½¶à£¬ةْ³ةµؤH2Oخïضتµؤء؟ش½´َ£¬CO2µؤخïضتµؤء؟ش½ةظ£¬ءيحâح¬خïضتµؤء؟µؤجحêب«ب¼ةص£¬؛ؤرُء؟ب،¾ِسعx+
ضµش½´َ£¬حêب«ب¼ةص؛ؤرُء؟ش½¶à£¬ةْ³ةµؤH2Oخïضتµؤء؟ش½´َ£¬CO2µؤخïضتµؤء؟ش½ةظ£¬ءيحâح¬خïضتµؤء؟µؤجحêب«ب¼ةص£¬؛ؤرُء؟ب،¾ِسعx+![]() µؤدà¶ش´َذ،£¬شٍµبخïضتµؤء؟µؤCH4؛حC3H8حêب«ب¼ةصت±£¬؛ؤرُء؟ا°صكذ،سع؛َصك£»µبضتء؟µؤCH4؛حC3H8حêب«ب¼ةصت±£¬؛ؤرُء؟ا°صك´َسع؛َصك£»بô؛ؤرُء؟دàح¬£¬¼´n(CH4)،ء£¨1+
µؤدà¶ش´َذ،£¬شٍµبخïضتµؤء؟µؤCH4؛حC3H8حêب«ب¼ةصت±£¬؛ؤرُء؟ا°صكذ،سع؛َصك£»µبضتء؟µؤCH4؛حC3H8حêب«ب¼ةصت±£¬؛ؤرُء؟ا°صك´َسع؛َصك£»بô؛ؤرُء؟دàح¬£¬¼´n(CH4)،ء£¨1+![]() £©=n(C3H8)،ء£¨3+
£©=n(C3H8)،ء£¨3+![]() £©£¬شٍ¶صكµؤخïضتµؤء؟ض®±بn(CH4)£؛n(C3H8)£½5:2£»
£©£¬شٍ¶صكµؤخïضتµؤء؟ض®±بn(CH4)£؛n(C3H8)£½5:2£»
II،¢H2،¢CO،¢CH4ئّجهب¼ةص£¬·¢ةْ·´س¦خھ£؛2H2+O2![]() 2H2O£¬2CO+O2
2H2O£¬2CO+O2![]() 2CO2£¬CH4+2O2
2CO2£¬CH4+2O2![]() CO2+2H2O£»
CO2+2H2O£»
£¨1£©بôAد´ئّئ؟µؤضتء؟شِ¼س£¬Bد´ئّئ؟µؤضتء؟²»±ن£¬Aضذخھإ¨ءٍثل£¬ثµأ÷Aخüتصءثث®£¬ض»؛¬سذH£¬²»؛¬C£¬زٍ¶ّئّجهXخھH2£¬¹ت´ً°¸خھ£؛H2£»
£¨2£©بôA،¢Bء½¸ِد´ئّئ؟µؤضتء؟¶¼شِ¼س£¬ثµأ÷ةْ³ةخïسذ¶رُ»¯ج¼،¢ث®£¬ثùزش؛¬سذC£¬H£¬Oشھثط£¬شٍ·´س¦خïضذز²ز»¶¨؛¬سذصâبضضشھثط£¬خïضتشع؟صئّضذب¼ةص£¬تا؛ح؟صئّضذµؤرُئّ·´س¦£¬رُئّسةOشھثط×é³ة£¬شٍC£¬H¶¨تا´سؤ³خïضتضذہ´£¬¶ّؤ³خïضتضذز²؟ةؤـ؛¬سذOشھثط£¬شٍئّجهX؟ةؤـتا£؛¢ظCH4،¢H2،¢CO£»¢عCH4£» ¢غCH4،¢H2£» ¢ـCH4،¢CO£» ¢فCO،¢H2،£
III،¢£¨1£©C5H12Oµؤ´¼£¬شعز»¶¨جُ¼دآؤـ·¢ةْ´ك»¯رُ»¯·´س¦£¬²ْخïؤـسëذآضئµؤاâرُ»¯ح·´س¦ةْ³ة؛ىة«³ءµي£¬ثµأ÷ء¬½سôا»ùµؤج¼ش×سةد؛¬سذء½¸ِاâش×س£¬´¼µؤ؛ث´إ¹²صٌاâئ×ضذسذب×é·ه£¬ثµأ÷¸أ´¼ضذ؛¬سذبضضاâش×س£¬ز»ضضHش×سشع-OHةد£¬ز»ضضHش×سشعء¬½سôا»ùµؤج¼ش×سةد£¬ز»ضضشعءيحâµؤ-CH3ةد£¬ثùزشئن½ل¹¹¼ٍت½خھ£¨CH3£©3CCH2OH£»
£¨2£©C5H12Oµؤ´¼£¬شعز»¶¨جُ¼دآ²»ؤـ·¢ةْدûب¥·´س¦£¬ثµأ÷سëôا»ùدàء¬µؤج¼ش×سµؤدàءعج¼ش×سةد²»؛¬Hش×س£¬شٍضذ¼نµؤج¼ش×سضذء¬½س3¸ِ-CH3؛حز»¸ِ-CH2OH£¬ثùزشئن½ل¹¹¼ٍت½خھ£؛£¨CH3£©3CCH2OH£»
£¨3£©·ض×ست½خھC5H12O£¬²»ؤـسë½ًتôؤئ·´س¦£¬ثµأ÷²»؛¬-OH£¬؛ث´إ¹²صٌاâئ×ضذسذثؤ×é·ه£¬ثµأ÷؛¬سذثؤضضاâش×س£¬·هµؤأو»±بخھ3£؛2£؛1£؛6£¬شٍثؤضضاâش×سµؤ¸ِت·ض±ًتا3،¢2،¢1،¢6£¬5¸ِج¼ش×س؛¬سذ4ضضHش×ساز²»؛¬ôا»ù£¬شٍأ؟¸ِج¼ش×سةد¶¼سذHش×س£¬ازئنضذء½¸ِ¼×»ùء¬½سشعح¬ز»¸ِج¼ش×سةد£¬ثùزشئن½ل¹¹¼ٍت½خھ£¨CH3£©2CH-O-CH2CH3،£