��Ŀ����
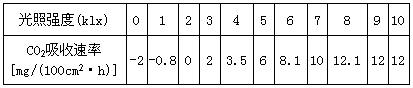
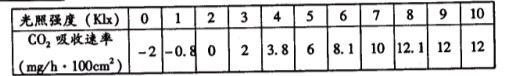
�±���ijֲ���ڲ�ͬ����ǿ�������ն�����̼�����ʣ�������ش�
| ����ǿ�ȣ�Klx�� | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| CO2�������ʣ�mg/h��100cm2�� | -2 | -0.8 | 0 | 2 | 3.8 | 6 | 8.1 | 10 | 12.1 | 12 | 12 |
��1������ǿ��Ϊ��ʱ������ֲ��CO2�ͷ�������Ҫ���������� _____��
��2���ڹ���ǿ�ȴﵽ8Klx��CO2���������������ǿ�����Ӷ����ӣ���Ҫ���ܻ�����_______________����Լ��
��3��������ǿ��Ϊ2Klxʱ��CO2������Ϊ�㡣��д������������̵��ܷ�Ӧʽ��
___________________________________________________
��4��ijУ�����о�С��������һ�������Ǻʲݵ����磬������������һ������ֲ��ʲ���һ������ֲ����Ǽƻ�ͨ��ʵ��̽����ǿ������������£�����ֲ�������ֲ��ҶƬ��Ҷ���غ����Ķ��١���������������ʵ����Ʒ�����
ʵ����裺����ֲ����ǿ��������ҶƬ��Ҷ���غ����϶࣬������ֲ��������������ҶƬ��Ҷ���غ����϶ࡣ
ʵ���þߣ��ڹ���������50���������Ҷ���غ�����Ҫ��ҩƷ������ȡ�
ʵ�鲽�裺
��_____________________________________________________________________
��_____________________________________________________________________
��_____________________________________________________________________
��_____________________________________________________________________
��������ۣ���________________________________________________________
__________________________________________________________�����������
��1���¶�
��2��CO2Ũ��
��3��CO2+H2O![]() ��CH2O��+O2
��CH2O��+O2
C6H12O6+6O2+6H2O![]() 6CO2+12H2O+������ֻ��һ��÷֣�
6CO2+12H2O+������ֻ��һ��÷֣�
��4��ʵ�鲽�裺
�ٽ������Ǻʲ������ƽ����Ϊ�������顣
�ڽ�����ֲ��ļ������綼���ڹ����ڸǣ�����ֲ����������綼�����ڹ����
�۽��������綼���ڽ�ǿ����������������
��һ��ʱ��ֱ�ⶨ����ֲ��ҶƬ��Ҷ���صĺ�����
��������ۣ�
�����ǵļ���ֲ��ҶƬ��Ҷ���غ������Ը������飬���ʲݵļ���ֲ��ҶƬ��Ҷ���غ������Ե������顣

��8�֣��±���ijͬѧ��������ʵ��ʱ���оٵ�ʵ����Ϻ�ʵ��������������ݣ���ݱ��ش��������⣺
| ��� | ���� | ʵ������ | �۲����� |
| A | ϡ���� | �Լ��� | ��ɫ��Ӧ |
| B | �˵Ŀ�ǻ��Ƥϸ�� | ������ | ϸ���ṹ�� |
| C | ��ɫ�����ƬҶ���Ƥ | 0.3 g/mL������Һ | �ʱڷ��� |
| D | H202��������ø | ��ͬ��pH | ʵ��������� |
| E | ��и��� | ����Һ��������ȾҺ�� | ϸ����Ⱦɫ�����Ŀ����̬ |
(2)A��ʵ�����õ��Լ�����___________���۲쵽����ɫ��_________��
(3)��C��ʵ������ʹ��0.3 g/mLKN03��Һ����ʵ��������_________________����2�֣�
(4)E��Ϊ�۲�ֲ��ϸ������˿����ʵ�飬ʵ����װƬ����������Ϊ_________________________��
��5��ijͬѧ��ɫ����ȡʵ����������ֹ��ɫ������ֽ�Ϸ����������ͼ��ʾ����ͼ��������Ҫ���պ���ɫ���� (�����)��2�֣���

�±���ijֲ���ڲ�ͬ�����¶Լ����������պ�Ũ�ȱ仯�����ֵ��������ñ������ܵó��Ľ�����
���� | NO | K+ | PO | H2O |
ҹ�����ֵ | 15�� | 30�� | 10�� | 10�� |
�������ֵ | 40�� | 15�� | 15�� | 50�� |
A��ˮ�����ε�������������Զ����Ĺ���
B�����ε����ն���������Ķ����й�
C��ˮ�����ε����տ��������¶��й�
D�������ֲ���ˮ��N�����ն��϶�
 ��������
��������