��Ŀ����
��6�֣���Ѫ��Ũ�������彡��״������Ҫָ��֮һ�����ּ��ز���Ѫ��Ũ�ȵĵ��ڡ�
��1��Ѫ��Ũ�ȵ�������Χ�� mg/dL���ȵ��ط��ڲ������������
���������� �����ϰ�������֬���͵����ʷֽ��ǿ�����»�
�����ݡ�Ѫ�Ǻ������͵��¾��ʺͻ���ʱ��Ӧ��ʱ�����߾�������
�Ի���֢״��
��2����ͼ�У��������߷ֱ��ʾ��ʳ��ѪҺ���ȵ��غ��ȸ�Ѫ������Ժ����ı仯�����б�ʾ�������ȵ��ر仯���Ƶ������� ����ʾ�������ȸ�Ѫ���ر仯���Ƶ������� ��

��3�����ʱ������Ѫ��Ӧ�ڿո�ʱ���У�������ѧ�������ų���ʳ�� �ĸ��š�
��8�֣�ө����ܷ�������Ϊө�������ͨ��ӫ����ø����ϵ�з�Ӧ���������������ӫ����ø������ֲ�����ڣ�Ҳ��ʹֲ���巢�⡣һֱ������ӫ����ø��Ψһ��Դ�Ǵ�ө��渹����ȡ�������������Ǵ�ѧ��һ���ѧ�ҳɹ���ͨ��ת���̽�ӫ����ø�����뵽�˾����ڣ����ڴ˾���������ӫ����ø������������е�֪ʶ�ش������й����⣺
��1���ڴ�ת�����У�Ŀ�Ļ����� ����ȡĿ�Ļ����;��ͨ������������ȡ��Ŀ�Ļ����;��������� ��
��2������Ŀ�Ļ����뵽�˾�������Ҫ������İ���������ѡ����ѡȡ�������ʱ����뿼�ǵ��� ��
A���ܹ�������ϸ���ڸ��Ʋ��ȶ����� B�������ض�������ø�е�
C��������Ŀ�Ļ�����ͬ�ļ��Ƭ�� D������ijЩ��ǻ���
��3�����п���Ϊ����ϸ���������� ��
A������ũ�˾� B����˸˾� C���ɾ��� D���ݲݸ˾�
��4���ڴ�ת�����У�����������Ϊ�����壻�ڽ���������DNA����˾�����֮ǰͨ��Ҫ�� �����˾���Ŀ���� ��ʹ��������ܹ���������ϸ������5������ӫ����ø���������ã�����һֱ���뽫�������Ϊʵ�鹤�ߣ�������ijһ����������һ��ͨ��ֲ���Ƿ���ȷ���û����Ƿ��Ѿ�ת�뵽ֲ�����ڣ����жϹ̵������Ƿ�ɹ�����ijֲ�����ڡ��������������ڵĹ̵�������ө������ڵ�ӫ����ø������ȣ����˼���Ե�˳����Ŀ��ͬ���⣬�ڽṹ���滹���ڲ�ͬ�㣬��Ҫ��ͬ�� ����ӫ����ø����ı������Ǽ�����������ġ�
��6�����ӽ����֡��ձ�������ȣ�ͨ��������������Ʒ�ֵ���Ҫ�ŵ���
��
��6�֣�
��1��80��120���Ǵ�л�������ǡ�
��2��b��c��
��3��Ѫ��Ũ��������
��8�֣�
��1��ӫ����ø�����˹��ϳɡ�
��2��ABD��
��3��AD��
��4��CaCl2������ϸ���ڵ�ͨ�ԡ�
��5���̵�����ı������������ġ�
��6��Ŀ����ǿ���������ڶ̣����Կ˷�ԶԴ�ӽ����͵��ϰ���
����������

 ���ɶ���ܲ��¿�ֱͨ�п�ϵ�д�
���ɶ���ܲ��¿�ֱͨ�п�ϵ�д�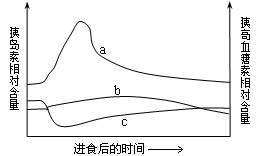


 ��1��Ѫ��Ũ�������彡��״������Ҫָ��֮һ�����ּ��ز���Ѫ��Ũ�ȵĵ��ڡ��ȵ��ط��ڲ������������������ �������������ϰ�������֬���͵����ʷֽ��ǿ�����»������ݡ�Ѫ�Ǻ������͵��¾��ʺͻ���ʱ��Ӧ��ʱ������ �����Ի���֢״��
��1��Ѫ��Ũ�������彡��״������Ҫָ��֮һ�����ּ��ز���Ѫ��Ũ�ȵĵ��ڡ��ȵ��ط��ڲ������������������ �������������ϰ�������֬���͵����ʷֽ��ǿ�����»������ݡ�Ѫ�Ǻ������͵��¾��ʺͻ���ʱ��Ӧ��ʱ������ �����Ի���֢״��