��Ŀ����
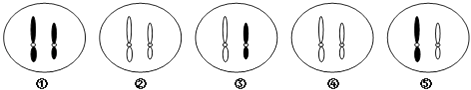
����Ŀ����ͼ��������ѧ��˹ͼ����1958�꽫���ܲ���Ƥ����һЩϸ����������ֲ��Ĺ���ʾ��ͼ�����ͼ�ش���������⣮
��1��[4]����[3]�е���ϸ�������γɵ�ϸ���ţ�ϸ�����ѷ�ʽ�� �� ��ϸ���ŵ��ص��� �� [4]�����γ�[5]������γ�һ��������ֲ�꣮
��2����һ��ʵ˵���߶ȷֻ���ֲ��ϸ���Ա����� ��
��3���ݴ�ʵ������ƶϣ��߶ȷֻ��Ķ���ϸ����Ҳ������������ö������Ƶĸ��壬ԭ���� ��
���𰸡�
��1���ѷֻ�����˿���ѣ��߶�Һ�ݻ�������̬�ı���ϸ�����ٷֻ�
��2��ϸ��ȫ����
��3��ϸ�����ں��б��ֱ������Ŵ����������ȫ���Ŵ�����
���������⣺��1��[4]��������֯����[3]�еĵ���ϸ�������ѷֻ��γɣ�ϸ�����ѷ�ʽ����˿���ѣ�������֯���ص��Ǹ߶�Һ�ݻ�������̬�ı���ϸ���������ٷֻ����̷�����[5]��״�壮��2����ֲ���ϸ���������������壬˵���߶ȷֻ���ֲ��ϸ���Ա�����ϸ��ȫ���Ե����ԣ���3��������ϸ����Ҳ����ȫ���ԣ���Ϊ����ϸ�����ں����Ŵ������ȫ���Ŵ����ʣ����Դ��ǣ���1���ѷֻ� ��˿���� �߶�Һ�ݻ�������̬�ı���ϸ�� �ٷֻ���2��ϸ��ȫ���ԣ�3��ϸ�����ں��б��ֱ������Ŵ����������ȫ���Ŵ�����
�����㾫����������Ҫ������ֲ��ϸ����ȫ���Լ�Ӧ�õ����֪ʶ�㣬��Ҫ����ֲ��ϸ����ȫ���ԣ�ֲ��ϸ��ȫ���Ե�ԭ��ֲ��ϸ���о��з��������������ȫ���Ŵ����ʲ�����ȷ�����⣮

 ��ʱѵ���������������ϵ�д�
��ʱѵ���������������ϵ�д� �ƸԾ���Ȥζ����ϵ�д�
�ƸԾ���Ȥζ����ϵ�д� ����С����ҵ��ϵ�д�
����С����ҵ��ϵ�д�����Ŀ���ش������й��������������õ�����:
KH2PO4 | 1.4 g |
Na2HPO4 | 2.1 g |
MgSO4��7H2O | 0.2 g |
FeCl3 | 0.1 g |
X | 1 g |
���� | �� |
��֬ | 15 g |
(1)���ϱ���ɸѡ������ϸ�����������䷽�������������Ͽ�������������____������,���гɷ�X����ΪĿ�ľ��ṩ��Դ��,�����ṩ________���Ʊ�����������һ�����˳���Ǽ����������___�������___����������������ij��÷�����_______��
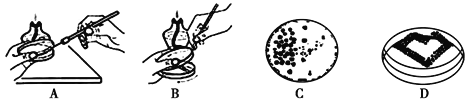
(2)��ͼA��B�Ǵ����������������ֽ��ַ���,C��D�ǽ��ֺ�������Ч����ijͬѧ������������ͼC��ʾЧ��,������õĽ��ַ�����[��]__([��]ѡ�A����B��);����ʱ���ܵ�ʧ������� ___��
(3)����ǿ������������ijЩ�����ܽ���ʯ�͡�����ʯ�͵��黯�ȡ�����ʯ���ȵ�ԭ��,ͨ�����;���ע�뺬�����ˮ����߲����ʵ��¼�����Ϊɸѡ�ʹ�����������,Ӧ�������������� ___��ΪΨһ̼Դ;����һ��ʱ��������������γɽ���Ȧ,��ʱѡȡ____�Ϳɻ�ø�Ч���ꡣ