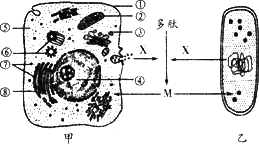
��Ŀ����
��ͼΪ����ϸ�����ڻ���֮������ʽ���ʾ��ͼ����ͼ�ش��������⣺
��1����ͼ��ʾϸ������Χ����֮��Ĺ�ϵ������ëϸѪ�ܹܱ�ϸ������ľ����ڻ�����________�������ţ�
��2��Ѫ������֯Һ���ܰ�����֮��������й�ϵ������һ������һ������£�����۳ɷ��ϵ���Ҫ��������________������ͬ��
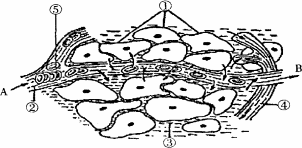
��3�������ͼΪ������֯�ֲ��ṹģʽͼ�����˴��ڼ���״̬ʱ��B����A��Һ����Ƚϣ�Ѫ�ǵĺ���B________������ڻ�С�ڣ�A��
��4��ͼ����ʾ�ڵ�ph����ά����________��ԭ�������к��е�________�����ʷ������õĽ����
�𰸣�
������
������
|
��1���ڢۡ���2�������ʡ���3�����ڡ���4��7.35��7.45��Na2CO3��H2CO3 |

��ϰ��ϵ�д�
 ��һ������ĩ�ٷֳ�̾�ϵ�д�
��һ������ĩ�ٷֳ�̾�ϵ�д�
�����Ŀ