��Ŀ����
����Ŀ��Ϊ�о��Ͳ���������BR����ֲ�����������ж������أ�IAA�������ã�������Ա�����Ͻ�Ϊ���Ͻ���
������ʵ�顣��ش��������⣺
��1��BR��Ϊһ��ֲ�D�أ���������ֲ�D�صĹ�ϵ��___________��
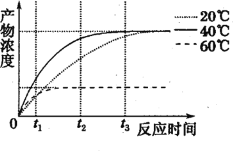
��2��������Ա�ںڰ���������Ұ���ͺ�BR�ϳ�ȱ��ͻ�������Ͻ��������ʵ�飬���������ˮƽ���ã�����һ��Ұ��������ʩ����ԴBR���������鲻ʩ�ӡ��ⶨ0~14h��������������������������ȣ��������ͼ��ʾ��
������������������������������ķ���______��ʩ����ԴBR��Ұ������������ᡢ����������������ȵ�ʱ����_________����BR�ϳ�ȱ��ͻ����ֲ�������������ȵ�ʱ����������������������ȵ�ʱ��_______������ʵ������˵��______________��
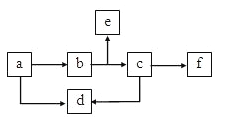
��3��IAA������Gø������Gø�ɴ���ɫ����������ɫ���������Ա��ת����Gø�����Ұ���ͺ�BR�ϳ�ȱ��ͻ����ֲ��������ú�����ɫ�������Һ����һ��ʱ��۲쵽Ұ����ֲ����������ɫ����ֲ��ڷ��������쳤������BR�ϳ�ȱ��ͻ����ֲ����������ɫ������ֲ��ڷ�������˵��_________�������Ʋ�BR�ܹ��ٽ�IAA�ļ������䡣
��4��Ϊ��֤�����Ʋ⣬�ɽ�һ����Ⲣ�Ƚ�Ұ���ͺ�BR�ϳ�ȱ��ͻ����ֲ������ϸ����IAA���������������ı���������______����֧�������Ʋ⡣
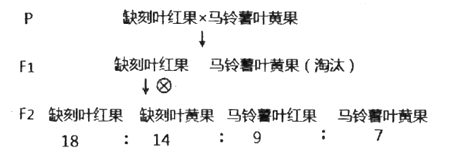
���𰸡����ּ�������ã���ͬ����ֲ����������� �� 4h �ͺ����� BR�ٽ�������������������� BR��Ӱ��IAA�ķֲ� Ұ����ֲ������ϸ���иû������������BR�ϳ�ȱ��ͻ����ֲ��
��������
������ͼ����ʵ������֪�����������������ķ����෴��ʩ����ԴBR��Ұ������������ᡢ�����ڵ�4Сʱ�Ϳɴﵽ��������ȣ�BR�ϳ�ȱ��ͻ���������������γɵ�ʱ������������ӳ٣�˵��BR�ٽ���������������ԣ�������������
��1����ֲ���������������Ӧ�����仯�Ĺ����У�����ֲ�D�ز����ǹ����������ã����Ƕ��ּ�������ù�ͬ���ڡ�
��2��ͨ��ͼʾ��֪�����������������ķ����෴����������������������������������ķ����ϣ�ʩ����ԴBR��Ұ�������������������������ڽ϶�ʱ���ڴﵽ��������ȣ�����������4hʱ�Ϳɴﵽ��������ȡ�BR�ϳ�ȱ��ͻ���������������γɵ�ʱ������������ӳ٣�������˵��BR���Դٽ����������������������
��3��������Ա��ת����Gø�����Ұ���ͺ�BR�ϳ�ȱ��ͻ����ֲ��������ú�����ɫ�������Һ����һ��ʱ��۲쵽Ұ����ֲ����������ɫ����ֲ��ڷ��������쳤������BR�ϳ�ȱ��ͻ����ֲ����������ɫ������ֲ��ڷ�������˵��BR��Ӱ��IAA�ķֲ��������Ʋ�BR�ܹ��ٽ�IAA�ļ������䡣
��4��Ϊ��֤BR�ܹ��ٽ�IAA�ļ��������Ʋ⣬�ɽ�һ����Ⲣ�Ƚ�Ұ���ͺ�BR�ϳ�ȱ��ͻ����ֲ������ϸ����IAA���������������ı���������Ұ����ֲ������ϸ���иû������������BR�ϳ�ȱ��ͻ����ֲ�꣬��˵��BR�ܹ��ٽ�IAA�ļ������䡣

 ����ͼ���������������ϵ�д�
����ͼ���������������ϵ�д� ����ѧҵ���Ե�����ϵ�д�
����ѧҵ���Ե�����ϵ�д�����Ŀ����Ҷ��ˮ�����й�����õ���Ҫ���٣���ֱ��������Ҷ��ˮƽ������Ҷ�������ͣ�����ͼ��ʾ��ijʵ��С������ͬ�����˵Ĺ��������£�����ֱ����Ҷ��ˮƽ��Ҷ�ľ�������ʡ�������(�����ų̶�)�Ͱ���CO2Ũ�ȣ�������±���ʾ����ش��������⣺
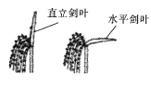
��������ʣ�molCO2m-2s-1�� | �����ȣ�molH2Om-2s-1�� | ����CO2Ũ�ȣ�molCO2mol -1�� | |
ֱ����Ҷ | 13 | 0.75 | 257 |
ˮƽ��Ҷ | 21 | 0.74 | 162 |
(1)ͬһˮ��ֲ���ϳ���ֱ����Ҷ��ˮƽ��Ҷ����Ҷ�ͣ�����ԭ����_______����ͼ������ˮƽ��Ҷ�Ĺ������ǿ�ȸ���ֱ����Ҷ�ģ�ԭ����___________________________��
(2)����CO2Ũ����ָϸ����϶�е�CO2Ũ�ȣ�����CO2����Ҷ��ϸ������_________(����)�б��̶���ֱ����Ҷ��ˮƽ��Ҷ�������ȼ���һ�£�����ˮƽ��Ҷ�İ���CO2Ũ����������ֱ����Ҷ�ģ�ԭ�������_____________________________________��
(3)����һ������ֱ����Ҷ��ˮƽ��Ҷ���ܹ�����ʣ���Ҫ�ⶨ��Ҷ��__________________��