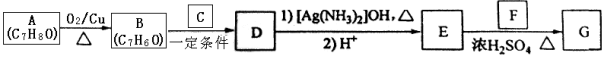��Ŀ����
����Ŀ���л���G������ʽΪC16H22O4���dz��õ�Ƥ�����������Ⱥϳɲ����е�������������һ�ֺϳ�·������ͼ��ʾ��

��֪���� A��һ�ֳ��������ĺ��������A������ͼ������ʺɱ�Ϊ46����˴Ź�������ͼ��������壬�ҷ����֮��Ϊ1��2��3 ��
�� E�DZ���ͬϵ���Է���������100��110֮�䣬��E�����ϵ�һ�ȴ���ֻ��2�֣�![]()
�� 1molF�������ı���NaHCO3��Һ��Ӧ�ɲ�������44.8L����״���£���
����R1��R2��ʾ��ԭ�ӻ���������
�����������Ϣ�ش��������⣺
��1��A������Ϊ_______��д��A ��һ��ͬ���칹��Ľṹ��ʽ_________��
��2��Aת��ΪB�Ļ�ѧ����ʽΪ__________________________��
��3��C�����������ŵ�����Ϊ_________
��4��E�Ľṹ��ʽΪ_____________��
��5��D��F��Ӧ����G�Ļ�ѧ��Ӧ����ʽΪ______________________��
��6��F�ж���ͬ���칹�壬д��ͬʱ��������������F��ͬ���칹����_____�֡�
�� �ܺ�NaHCO3��Һ��Ӧ���� �ܷ���������Ӧ���� ��FeC13��Һ����ɫ��
��7����������������Ϣ�ͼ�֪��Ϣ��������Ҵ��ͱ���Ϊԭ�ϣ����Լ���ѡ���Ʊ�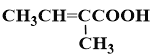 �ĺϳ�·��_______________���ϳ�·������ͼʾ�����£�
�ĺϳ�·��_______________���ϳ�·������ͼʾ�����£�
���𰸡� �Ҵ� CH3OCH3 2CH3CH2OH+O2![]() 2CH3CHO+2H2O ̼̼˫����ȩ��
2CH3CHO+2H2O ̼̼˫����ȩ�� ![]()
![]() 10
10 
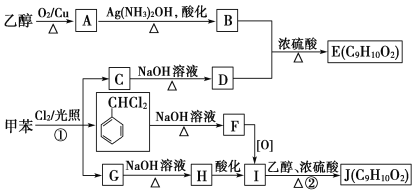
��������A��һ�ֳ��������ĺ��������A������ͼ������ʺɱ�Ϊ46����˴Ź�������ͼ��������壬�ҷ����֮��Ϊ1:2:3��AΪCH3CH2OH���Ҵ�������������ȩ��������Ϣ����ȩ���������Ƽ���ʱ����C��CΪCH3CH=CHCHO��C�������ӳ�����D��DΪCH3CH2CH2CH2OH��E�DZ���ͬϵ���Է���������10��110֮�䣬��������F��1molF�������ı���NaHCO3��Һ��Ӧ�ɲ���2mol������̼����F�к���2���Ȼ������EΪ���ױ���E�����ϵ�һ�ȴ���ֻ��2�֣�EΪ![]() ����FΪ
����FΪ![]() ��
��
(1)��������������AΪCH3CH2OH���������Ҵ����Ҵ�����ѻ�Ϊͬ���칹�壬�ʴ�Ϊ���Ҵ��� CH3OCH3��
(2)�Ҵ�������������ȩ����Ӧ�Ļ�ѧ����ʽΪ2CH3CH2OH+O2![]() 2CH3CHO+2H2O���ʴ�Ϊ��2CH3CH2OH+O2
2CH3CHO+2H2O���ʴ�Ϊ��2CH3CH2OH+O2![]() 2CH3CHO+2H2O��
2CH3CHO+2H2O��
(3)CΪCH3CH=CHCHO��C��������������̼̼˫����ȩ�����ʴ�Ϊ��̼̼˫����ȩ����
(4)EΪ![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
(5)DΪCH3CH2CH2CH2OH��FΪ![]() ������������Ӧ����G�Ļ�ѧ��Ӧ����ʽΪ
������������Ӧ����G�Ļ�ѧ��Ӧ����ʽΪ![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
(6)FΪ![]() ���� �ܺ�NaHCO3��Һ��Ӧ��˵�������Ȼ����� �ܷ���������Ӧ��˵������ȩ����Ϊ���������� ��FeC13��Һ����ɫ��˵�����з��ǻ�������������F��ͬ���칹����
���� �ܺ�NaHCO3��Һ��Ӧ��˵�������Ȼ����� �ܷ���������Ӧ��˵������ȩ����Ϊ���������� ��FeC13��Һ����ɫ��˵�����з��ǻ�������������F��ͬ���칹����![]() ��
��![]() (����λ�ÿɱ�)�ȣ��ʴ�Ϊ��
(����λ�ÿɱ�)�ȣ��ʴ�Ϊ��![]() ��
��![]() ��
��
(7)���Ҵ��ͱ���Ϊԭ���Ʊ�![]() �����Խ��Ҵ�����������ȩ���������������ɱ�ȩ��������Ϣ��������CH3CH=C(CH3)CHO,����������������������Ϊ
�����Խ��Ҵ�����������ȩ���������������ɱ�ȩ��������Ϣ��������CH3CH=C(CH3)CHO,����������������������Ϊ![]() ������ͼΪ
������ͼΪ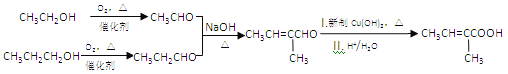 ���ʴ�Ϊ��
���ʴ�Ϊ��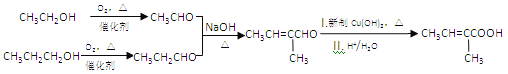 ��
��