��Ŀ����
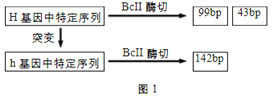
����Ŀ��ij�²�����h����������H�е�ijһ�ض����о�Bc��ø�кɲ�����С��ͬ��Ƭ�Σ���ͼ1��bp��ʾ����ԣ����ݴ˿ɽ��л�����ϣ�ͼ2Ϊij������ڸò����Ŵ�ϵ��ͼ��ͼ3Ϊ��-1����-2����-1�ŵ���ϸ��������Ͻ����
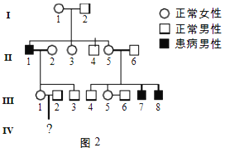
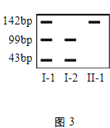
��1������ͼ3������Ϸ�������-1�ŵ��²�����������________�������Ŵ������Ŵ���ʽ��________��
��2����-5�ŵĻ�������г���99bp�ĸ�����________����-5�Ļ�������г���142bp�ĸ�����_______��
��3����-1����-2��������ĺ��ӽ��л�����ϣ����ܳ���ͼ3�е�________�������
��4����������Ϸ�������-1����-2�ĸ�ĸ��������h������ô�ü���h����������________��ͼ2��ʾ��-1�ŵ�h����һ�����Ŵ�����������·�����ȷ����________
A��h������������ܾ��ѷ��ѷֻ�����Ϊ��-1�ŵĹ����в���
B����-1�ŵ���ԭϸ�������γɵ�������ϸ��������h����
C��h�������������-1����ԭϸ��������һ�η��Ѽ��ڲ���
D����-4����-5��������Ϊ��-1�Ų����ܾ����Dz���h�������ϸ��
���𰸡�I-1 ��X�����Ŵ���X���������Ŵ��� 1��100%�� 1/2 3 I-1�����˻���ͻ�� A
��������
����ͼ1������H����ø�к�ɲ���99bp��43bp���ֳ��ȵ�Ƭ�Σ�������h����ø�к�ֻ�ܲ���142bpһ�ֳ��ȵ�Ƭ�Ρ�
����ͼ2��ͼ3����-1����-2������������-1�Ż�����������������Ϊ���ԡ���˵���ò��������Ŵ���������ͼ3��֪��-1���л���H�ͻ���h������-2ֻ������H��˵���ò�Ϊ��XȾɫ�������Ŵ�����
��1�������Ϸ�����֪���ò�Ϊ��XȾɫ�������Ŵ����������-1�ŵ��²�����������I-1��
��2����-5�ŵĻ�����XHX_�����л���H����ø�к�ɲ���99bp��������������г���99bp�ĸ�����100%������h����ø�к�����142bp������-5�Ļ����ͼ�������1/2XHXH��1/2XHXh��������������г���142bp�ĸ�����1/2��
��3����-1�Ļ�����ΪXHXh����-2�Ļ�����ΪXHY�����߽���������ӵĻ������Ϊ��XHXH��XHXh��XHY��XhY������XHXh���л�����Ͽɳ���ͼ3�еĵ�һ�����������XHXH��XHY���л�����ϿƳ���ͼ3�еĵ�3���������XhY���л�����ϿƳ���ͼ3�еĵ�2�������
��4����������Ϸ�������-1����-2��XHY���ĸ�ĸ��������h������ô�ü���h����������I-1�����˻���ͻ�䡣
�⣺AC����-1����������Ϻ���H��h���ֻ����������ΪXHXh����h����һ�����Ŵ��������ԭ����h������������ܾ��ѷ��ѷֻ�����Ϊ��-1�ŵĹ����в�����A��ȷ��C����
B����-1�ŵĻ�����ΪXHXh������ԭϸ��������h�������γɵ���ϸ���к���h����ĸ���Ϊ1/2��B����
D����-4��������Ϊ��-1�Ų����ܾ����Dz���h�������ϸ������-5��������Ϊ��-2�Ų����ܾ����Dz���h����ľ��ӣ�D����
��ѡA��

 ��У����ϵ�д�
��У����ϵ�д�����Ŀ��ij������ֲ��ΪXY���Ա�������仨����ɫ�ش�л��ͼ������A��a����λ�ڳ�Ⱦɫ���ϡ�����ɫ�����ɫ��ͬʱ����Ϊ�ϻ�����������ijһ���Ա𣬶���һ�Ա�ĸ�������������һ�Դ����ױ��ӽ����õ�F1��F1�д���ֲ���ӽ����õ�F2����������ʾ������˵��������ǣ� ��

�ױ� | F1 | F2 |
�ƣ����� | �ƣ����� | �ƣ�6������2�� |
�ۣ��컨 | �ۣ����� | �ۣ�3������1�컨��1�� |
A.�������ϻ�ֲ����Ա�������
B.F1�Ļ�������AaXBXb��AaXBY
C.��ȡF2�еİ�ֲ������䣬�Ӵ��к컨�ĸ�����7/8
D.�������������˶�Ի�����Կ���һ����״