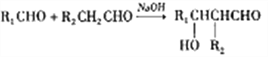��Ŀ����
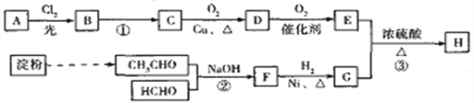
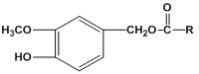
����Ŀ���������������Ļ��Գɷ֣�����Ԥ�����ಡ�� Ҳ�ܻ��⼡��ؽ���ʹ�����������������Ľṹ���Ա�ʾΪ�� (R Ϊ����)������һ���������������J �ĺϳ�·�����£�
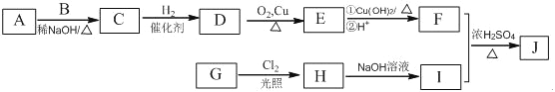
��֪��
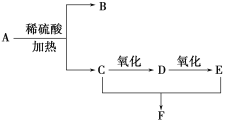
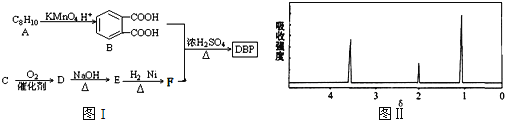
�� A��B ��E Ϊͬϵ�����B ����Է�������Ϊ44��A ��B �˴Ź���������ʾ��������壻
��������J �ķ���ʽΪC15H22O4��
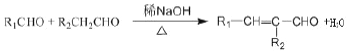
��
�ش��������⣺
��1��G ���������ŵ�����Ϊ___________��
��2����A ��B ����C �Ļ�ѧ����ʽΪ___________��
��3����C ����D �ķ�Ӧ����Ϊ___________��D �Ļ�ѧ����Ϊ___________��
��4����H ����I �Ļ�ѧ����ʽΪ___________��
��5��J �Ľṹ��ʽΪ___________��
��6��G ��ͬ���칹���У������ϵ�һ�ȴ���ֻ��һ�ֵ����� ��(���������칹)���˴Ź���������ʾ2 ������ (д�ṹ��ʽ)��
���𰸡���1���Ѽ���(��)�ǻ�����2��(CH3)3CCHO +CH3CHO![]() (CH3)3CCH =CHCHO
(CH3)3CCH =CHCHO
��3���ӳ�(��ԭ)��4��4-����-1-�촼��
��4�� ![]() +2NaOH
+2NaOH![]()
![]() +NaCl+H2O��
+NaCl+H2O��
��5��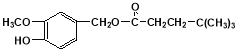 ����6��8������
����6��8������
��������
���������������J�ķ���ʽΪC15H22O4�����J�ĽṹΪ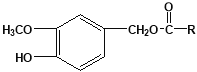 ����RΪ��C6H13�����F��IΪ
����RΪ��C6H13�����F��IΪ ��C6H13COOH���������ͼ֪��FΪC6H13COOH��IΪ
��C6H13COOH���������ͼ֪��FΪC6H13COOH��IΪ![]() �����GΪ
�����GΪ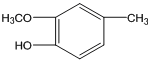 ��HΪ
��HΪ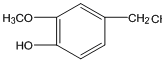 ��B����Է�������Ϊ44��B�˴Ź���������ʾ�����������BΪCH3CHO��A��BΪͬϵ���������Ϣ�ۣ�AΪC4H9CHO��A�ĺ˴Ź���������ʾ�����������AΪ(CH3)3CCHO��CΪ(CH3)3CCH =CHCHO��DΪ(CH3)3CCH 2CH2CH2OH��EΪ(CH3)3CCH 2CH2CHO�� FΪ(CH3)3CCH 2CH2COOH��
��B����Է�������Ϊ44��B�˴Ź���������ʾ�����������BΪCH3CHO��A��BΪͬϵ���������Ϣ�ۣ�AΪC4H9CHO��A�ĺ˴Ź���������ʾ�����������AΪ(CH3)3CCHO��CΪ(CH3)3CCH =CHCHO��DΪ(CH3)3CCH 2CH2CH2OH��EΪ(CH3)3CCH 2CH2CHO�� FΪ(CH3)3CCH 2CH2COOH��
��1��GΪ �����������ŵ����Ѽ���(��)�ǻ���
�����������ŵ����Ѽ���(��)�ǻ���
��2��AΪ(CH3)3CCHO��BΪCH3CHO��A��B����C�Ļ�ѧ����ʽΪ(CH3)3CCHO +CH3CHO![]() (CH3)3CCH =CHCHO��
(CH3)3CCH =CHCHO��
��3��CΪ(CH3)3CCH =CHCHO��DΪ(CH3)3CCH 2CH2CH2OH��C�����������ӳɷ�Ӧ����D��DΪ(CH3)3CCH2CH2CH2OH������Ϊ4��4-����-1-�촼��
��4�� ������������Һ�з���ˮ�ⷴӦ����I����ѧ����ʽΪ
������������Һ�з���ˮ�ⷴӦ����I����ѧ����ʽΪ![]() +2NaOH
+2NaOH ![]()
![]() +NaCl+H2O��
+NaCl+H2O��
��5����������������JΪ ��
��
��6��GΪ ��G��ͬ���칹���У������ϵ�һ�ȴ���ֻ��һ�ֵ���
��G��ͬ���칹���У������ϵ�һ�ȴ���ֻ��һ�ֵ���![]() ��
��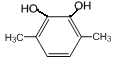 ��
��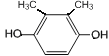 ��
��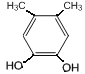 ��
��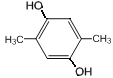 ��
��![]() ��
��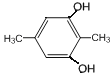 ��
��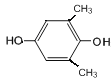 ��8�֡�
��8�֡�

 ��ѧ��ʦ����ϵ�д�
��ѧ��ʦ����ϵ�д�