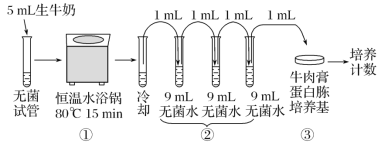
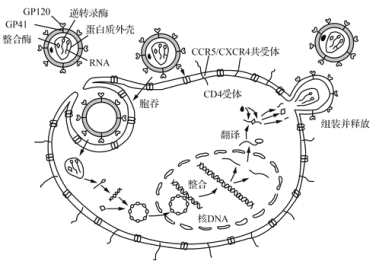
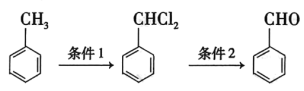
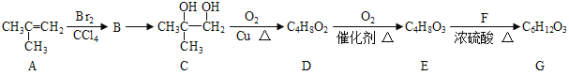��Ŀ����
����Ŀ��1mol�������ΪC3H8O��Һ̬�л���A������������������ʱ��������11.2L����״���£��������Իش��������⣺
��1��A�����б���һ��__������֪�˻�����̼����һ�ˣ���A����֧������A�Ľṹ��ʽΪ__��
��2��A��Ũ���Ṳ�ȣ���������ȥ1����ˮ����B����B�Ľṹ��ʽΪ__����Bͨ����ˮ���ܷ���___��Ӧ����C����C�Ľṹ��ʽΪ___��
��3��A��ͭ������ʱ�����������ȷ���������Ӧ����D����D�Ľṹ��ʽΪ___��
��4��д������ָ����Ӧ�Ļ�ѧ����ʽ��
��A��B___��
��B��C___��
��A��D___��
���𰸡��� CH3CH2CH2OH CH3CH=CH2 �ӳ� CH3CHBrCH2Br CH3CH2CHO CH3CH2CH2OH![]() CH3CH=CH2��+H2O CH3CH=CH2+Br2��CH3CHBrCH2Br 2CH3CH2CH2OH+O2
CH3CH=CH2��+H2O CH3CH=CH2+Br2��CH3CHBrCH2Br 2CH3CH2CH2OH+O2![]() 2CH3CH2CHO+2H2O
2CH3CH2CHO+2H2O
��������
1molC3H8O���Ʒ�Ӧ����n(H2)=![]() =0.5mol����C3H8O����1���ǻ������ǻ���̼����һ�ˣ���A����֧������AӦΪCH3CH2CH2OH����Ũ���������¿�����CH3CH=CH2��CH3CH=CH2����ˮ�����ӳɷ�Ӧ����CH3CHBrCH2Br��CH3CH2CH2OH����������������CH3CH2CHO���ݴ˽��
=0.5mol����C3H8O����1���ǻ������ǻ���̼����һ�ˣ���A����֧������AӦΪCH3CH2CH2OH����Ũ���������¿�����CH3CH=CH2��CH3CH=CH2����ˮ�����ӳɷ�Ӧ����CH3CHBrCH2Br��CH3CH2CH2OH����������������CH3CH2CHO���ݴ˽��
(1)1molC3H8O���Ʒ�Ӧ����n(H2)=![]() =0.5mol����C3H8O����1���ǻ������ǻ���̼����һ�ˣ���A����֧������AӦΪCH3CH2CH2OH��
=0.5mol����C3H8O����1���ǻ������ǻ���̼����һ�ˣ���A����֧������AӦΪCH3CH2CH2OH��
(2)��Ũ���������£�CH3CH2CH2OH������ȥ��Ӧ����B�� A��B��Ӧ�ķ���ʽΪ��CH3CH2CH2OH![]() CH3CH=CH2��+H2O��BΪCH3CH=CH2������ˮ�����ӳɷ�Ӧ����C��B��C��Ӧ�ķ���ʽΪ��CH3CH=CH2+Br2��CH3CHBrCH2Br��CΪ CH3CHBrCH2Br��
CH3CH=CH2��+H2O��BΪCH3CH=CH2������ˮ�����ӳɷ�Ӧ����C��B��C��Ӧ�ķ���ʽΪ��CH3CH=CH2+Br2��CH3CHBrCH2Br��CΪ CH3CHBrCH2Br��
(3)CH3CH2CH2OH����ͭ������ʱ��������һ����ȣ�����������Ӧ����DΪ��CH3CH2CHO��A��D��Ӧ�ķ���ʽΪ��2CH3CH2CH2OH+O2![]() 2CH3CH2CHO+2H2O��
2CH3CH2CHO+2H2O��
(4)�����Ϸ�����֪�йػ�ѧ����ʽΪ��
A��B��CH3CH2CH2OH![]() CH3CH=CH2��+H2O��
CH3CH=CH2��+H2O��
B��C��CH3CH=CH2+Br2��CH3CHBrCH2Br��
A��D��2CH3CH2CH2OH+O2![]() 2CH3CH2CHO+2H2O��
2CH3CH2CHO+2H2O��