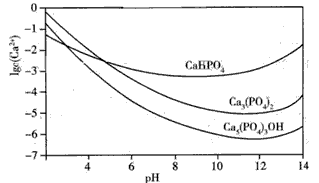
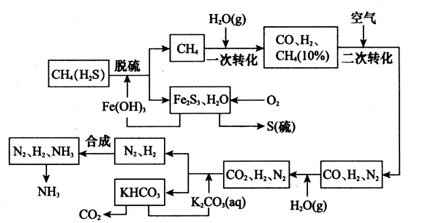
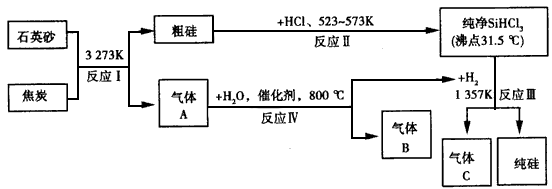
��Ŀ����
����ѡһ��ѡ��ѧ�뼼����
��������(ClO2)���������Ĵ�ɱ������������ҵ����NaCl ��ԭNaClO3����ClO2�Ĺ���������ͼ��ʾ��
��������(ClO2)���������Ĵ�ɱ������������ҵ����NaCl ��ԭNaClO3����ClO2�Ĺ���������ͼ��ʾ��
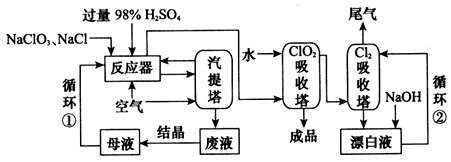
(1)��Ӧ���з����Ļ�ѧ��Ӧ����ʽΪ___________ ����Ӧ�������ɵ�ClO2��Cl2 ��ͨ�������������ClO2�������������Ļ��Һ�������������������ų���Һ�����ʵijɷ���Ҫ��________________ ���ѧʽ����
(2)�����н�NaClO3��NaCl�����ʵ���֮��1��1.05�Ļ� ��ˮ��Һ���뷴Ӧ����NaCl�Թ�����Ŀ����____________��
(3)��ȡClO2���������ӽ���Ĥ�ָ��ĵ��ص��NaClO2��Һ���������Ҽ����������___________���������Ҳ�����������________���������ӷ���ʽ�ɱ�ʾΪ______��
(2)�����н�NaClO3��NaCl�����ʵ���֮��1��1.05�Ļ� ��ˮ��Һ���뷴Ӧ����NaCl�Թ�����Ŀ����____________��
(3)��ȡClO2���������ӽ���Ĥ�ָ��ĵ��ص��NaClO2��Һ���������Ҽ����������___________���������Ҳ�����������________���������ӷ���ʽ�ɱ�ʾΪ______��
(1)2NaClO3+2NaCl+2H2SO4=2ClO2��+ Cl2��+2Na2SO4+2H2O ��Na2SO4��H2SO4��NaCl
(2)ʹNaClO3��ַ�Ӧ�������NaClO3�������ʣ�
(3) NaClO2��Һ ��H2��NaOH ��2ClO2-+2H2O 2ClO2��+H2��+2OH-
2ClO2��+H2��+2OH-
(2)ʹNaClO3��ַ�Ӧ�������NaClO3�������ʣ�
(3) NaClO2��Һ ��H2��NaOH ��2ClO2-+2H2O
 2ClO2��+H2��+2OH-
2ClO2��+H2��+2OH- 
��ϰ��ϵ�д�
�����Ŀ