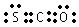��Ŀ����
��12�֣���֪�ɶ�����Ԫ����ɵ����ֳ������������A��B��C��D��E��������ԭ����Ŀ����Ϊ2��3��4��5��6������A��B��E����18�����ӣ�C��D����10�����ӡ���ش�
��1��D�к��еĻ�ѧ�������� ����ʵ������25�桢101kPaʱ��8gD��O2��ȫȼ�գ������ȶ�������ʱ�ų�445kJ����������÷�Ӧ���Ȼ�ѧ����ʽΪ ��
��2�������£���һ����C��ϡ��Һ����μ���A��ϡ��Һ�������ӻ�����XY4Z��X��Y��Z��ʾԪ�ط��ţ����ɣ����ҺpH�仯��ͼ��ʾ��ʵ������У���pH=7ʱ�����й��ڻ��Һ������Ũ���ж���ȷ���� ��

��. c(XY+4)>c(Z��)>c(OH��)=c(H+)
��. c(XY+4)=c(Z��)��c(OH��)=c(H+)
��. c(Z��)>c(XY+4)> c(OH��)=c(H+)
��. c(XY+4) + c(H+) = c(Z��) + c(OH��)
��3��XY4Z��ҺPH 7���С�ڡ����ڡ����ڡ�������ԭ�������ӷ���ʽ��ʾΪ�� ��
��4����B����ͨ��Cu��OH��2����Һ�У����DZ�Ϊ��ɫ��ԭ���� ��
��5��E�ĺ˴Ź�������ͼ��������壬�ҷ帲�ǵ������Ϊ3�U1 ��E�ĽṹʽΪ�� ��
��1��D�к��еĻ�ѧ�������� ����ʵ������25�桢101kPaʱ��8gD��O2��ȫȼ�գ������ȶ�������ʱ�ų�445kJ����������÷�Ӧ���Ȼ�ѧ����ʽΪ ��
��2�������£���һ����C��ϡ��Һ����μ���A��ϡ��Һ�������ӻ�����XY4Z��X��Y��Z��ʾԪ�ط��ţ����ɣ����ҺpH�仯��ͼ��ʾ��ʵ������У���pH=7ʱ�����й��ڻ��Һ������Ũ���ж���ȷ���� ��

��. c(XY+4)>c(Z��)>c(OH��)=c(H+)
��. c(XY+4)=c(Z��)��c(OH��)=c(H+)
��. c(Z��)>c(XY+4)> c(OH��)=c(H+)
��. c(XY+4) + c(H+) = c(Z��) + c(OH��)
��3��XY4Z��ҺPH 7���С�ڡ����ڡ����ڡ�������ԭ�������ӷ���ʽ��ʾΪ�� ��
��4����B����ͨ��Cu��OH��2����Һ�У����DZ�Ϊ��ɫ��ԭ���� ��
��5��E�ĺ˴Ź�������ͼ��������壬�ҷ帲�ǵ������Ϊ3�U1 ��E�ĽṹʽΪ�� ��
��12�֣�
��1�����ۼ���1�֣�
CH4(g)+2O2(g)=CO2(g)+2H2O(l) ��H=��890kJ��mol��1��2�֣�
��2������2�֣�
��3��С�ڣ�1�֣�NH+4 + H2O NH3��H2O + H+ ��2�֣�
NH3��H2O + H+ ��2�֣�
��4��CuS���ܽ��С��Cu��OH��2���ܽ�ȣ�2�֣�
��5��CH3-OH��2�֣�
��1�����ۼ���1�֣�
CH4(g)+2O2(g)=CO2(g)+2H2O(l) ��H=��890kJ��mol��1��2�֣�
��2������2�֣�
��3��С�ڣ�1�֣�NH+4 + H2O
 NH3��H2O + H+ ��2�֣�
NH3��H2O + H+ ��2�֣���4��CuS���ܽ��С��Cu��OH��2���ܽ�ȣ�2�֣�
��5��CH3-OH��2�֣�
��

��ϰ��ϵ�д�
�����Ŀ
 ��
��