��Ŀ����
��ͼ��һ������Ͷ�������Ĵ������ϵͳ��ԭ��ͼ������е���������Ϊ�缫���м�Ϊ����ѡ���Ե�أ��䡢�ŵ�Ļ�ѧ��Ӧ����ʽΪ2Na2S2+NaBr3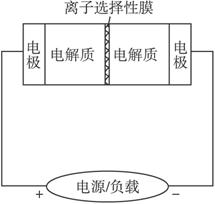
A.���Ĺ����е�0.1 mol Na+ͨ�����ӽ���Ĥʱ������ͨ��0.1 mol����
B.��طŵ�ʱ��������ӦΪ��3NaBr-2e-====NaBr3+2Na+
C.�������������Ӵ��ҵ���ͨ�����ӽ���Ĥ
D.�ŵ�����������Ӵ��ҵ���ͨ�����ӽ���Ĥ
���������⿼���˵绯ѧԭ����A.����ת�����غ�ģ���ȷ��B.�ŵ�ʱNaBr3+2e-+2Na+ ====3NaBr������B����C.���ʱNa+��������������������C����D��ȷ��
�𰸣�AD

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ

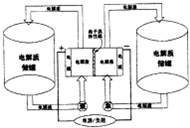 ��ͼ��һ������Ͷ�������Ĵ������ϵͳ����������Ϊ����ʴ��ޣ�����Ϊ��أ������ͨ���ò����ڴ��͵�ؼ�ѭ��������е���������Ϊ�缫���м�Ϊ����ѡ����Ĥ���ڵ�طŵ�ͳ��ʱ��Ĥ������������ͨ�����ŵ�ǰ����Ĥ�����ĵ����ΪNa2S2��NaIX��3��x��5�����ŵ�ֱ��ΪNa2S4��NaI��������˵������ȷ���ǣ�������
��ͼ��һ������Ͷ�������Ĵ������ϵͳ����������Ϊ����ʴ��ޣ�����Ϊ��أ������ͨ���ò����ڴ��͵�ؼ�ѭ��������е���������Ϊ�缫���м�Ϊ����ѡ����Ĥ���ڵ�طŵ�ͳ��ʱ��Ĥ������������ͨ�����ŵ�ǰ����Ĥ�����ĵ����ΪNa2S2��NaIX��3��x��5�����ŵ�ֱ��ΪNa2S4��NaI��������˵������ȷ���ǣ�������

