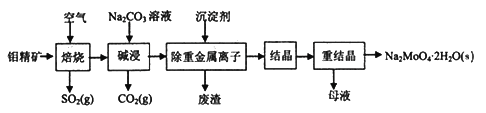
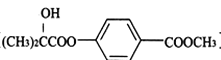
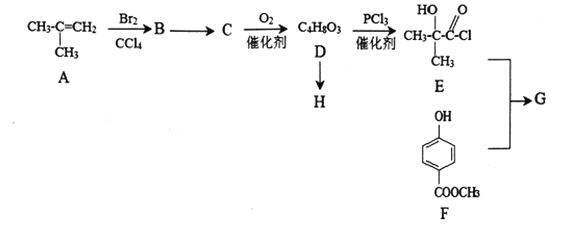
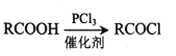
��Ŀ����
����Ŀ��ijͬѧ�����Ʒ���������������Һ�����п���ʵ�ֵ���
A. ֻ��0.1 mol Na+��0.2mol Mg2����0.1 mol Cl-��0.1 mol NO3-����Һ
B. ֻ��0.1mol NH4+��0.1 mol Ca2����0.1 mol CO32-��0.1mol Cl-����Һ
C. �ڱ�״���£���VL���壨Ħ��������M g/mol������0.1Lˮ�У�������Һ���ܶ�Ϊd g/mL�������Һ�����ʵ���Ũ��Ϊ1000Vd/��2240+VM��mol/L
D. ����1000mL����ƿ���ձ�������������Ͳ��58.5gNaCl�����ˮ����1L1mol/L��NaCl��Һ
���𰸡�C
��������
A����ҺҪ�ʵ�����,���������������������Ҫ�������������ĸ���������,��ֻ��0.1 mol Na+��0.2mol Mg2����0.1 mol Cl-��0.1 mol NO3-����Һ��,����������������ɵ����ʵ���n=0.1mol+0.2mol��2=0.5mol,�����������ĸ���ɵ����ʵ���n=0.1mol+0.1mol=0.2mol,��Һ���ʵ���������A����;
B������Һ��, Ca2���� CO32-��Ӧ����̼��Ƴ���������Ca2���� CO32-���ܹ�������B����;
C���ڱ�״���£���VL���壨Ħ��������M g/mol������0.1Lˮ�У����ʵ����ʵ���ΪVL��22.4L/mol=V/22.4mol����Һ�����ΪV=m/![]() =(V/22.4mol��M g/mol+100g)/d g/mL�����Դ���Һ�����ʵ���Ũ��ΪV/22.4mol��[1000Vd/(V/22.4mol��M g/mol+100g)/d g/mL]=��2240+VM��mol/L,��C��ȷ��
=(V/22.4mol��M g/mol+100g)/d g/mL�����Դ���Һ�����ʵ���Ũ��ΪV/22.4mol��[1000Vd/(V/22.4mol��M g/mol+100g)/d g/mL]=��2240+VM��mol/L,��C��ȷ��
D������1L1mol/L��NaCl��Һ�ڶ���ʱ��Ҫ�õ���ͷ�ι�,��������ʱ��Ҫ�õ���ƽ����D����.
��������������ӦѡC��