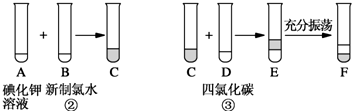
��Ŀ����
��ͼ��ʾ����֪��
�� �ټס��ҡ�����Ϊǰ������Ԫ�ص����嵥�ʣ���Ϊ���嵥�ʡ�
�� ����һ�������¼���������붡������������l��3���X��Y���ڲ�����Ԫ�ؼ׳ʸ��ۡ�
�� ����һ������������������붡�������ʵ���֮��1��2��Ӧ���ֱ�����Z��W���ڲ�����Ԫ���ҳʸ��ۡ�
����գ�
(1)����_______________________������_______________________��
(2)д��X��Y�ĵ���ʽ
________________________________________��_________________________________��
(3)���붡��Ӧ����W�Ļ�ѧ����ʽ��
________________________________________________________________��
(4)�������Ӧ����X�Ļ�ѧ����ʽ��
________________________________________________________________��
(5)ʵ������ȡX�Ļ�ѧ����ʽ��
________________________________________________________________��
�������� (1) N2 (1��) O2 (1��)��
���� (2) �� ��(��2��)����������������������
(3) O2 + 2Mg��2MgO�� (2��)
��4��N2 + 3H2��= 2NH3(2��) ��5��2NH4Cl + Ca(OH)2 2NH3��+ CaCl2 + 2H2O (2��)

 ��ĩ���䵥Ԫ�����ิϰ��ϵ�д�
��ĩ���䵥Ԫ�����ิϰ��ϵ�д� �����£�CaSO4��ˮ�еij����ܽ�ƽ��������ͼ��ʾ����֪Ksp��CaSO4��=9��10-6������˵����ȷ���ǣ�������
�����£�CaSO4��ˮ�еij����ܽ�ƽ��������ͼ��ʾ����֪Ksp��CaSO4��=9��10-6������˵����ȷ���ǣ�������| A��a���Ӧ��KspС��c���Ӧ��Ksp | B��a���Ϊb�㽫�г������� | C����������ˮ����ʹc���Ϊd�� | D�����д���SO42-����Һ�п϶�������Ca2+���� |
| X | Y |
| Z |
| A����һ�����ܣ�X��Y |
| B��X���ʵĻ�ѧ���ʱ�Z���ʻ��� |
| C��X��Y���γɶ��ֻ��������XY2���Ӽ������� |
| D��X�ij����⻯����ȶ��Ա�Z���⻯��ǿ |
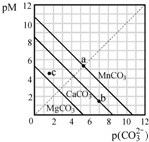 ��2013?���գ�һ���¶��£�����̼����MCO3��M��Mg2+��Ca2+��Mn2+���ij����ܽ�ƽ��������ͼ��ʾ����֪��pM=-lgc��M����pc��CO32-��=-lgc��CO32-��������˵����ȷ���ǣ�������
��2013?���գ�һ���¶��£�����̼����MCO3��M��Mg2+��Ca2+��Mn2+���ij����ܽ�ƽ��������ͼ��ʾ����֪��pM=-lgc��M����pc��CO32-��=-lgc��CO32-��������˵����ȷ���ǣ�������