��Ŀ����
��֪2H2(g)+O2(g) ��2H2O(l��+571.6kJ��2H2(g)+O2(g) ��2H2O(g)+483.6kJ������˵����ȷ����
- A.2 molH2(g)��1molO2(g)����������2mol H2O (l)������
- B.1 mol H2O (g)�ֽ��H2(g)��O2 (g)������241.8kJ����
- C.1mol H2O (l)���1mo1 H2O (g)������88 kJ����
- D.��������H2O (g)��H2O(l������������
B
���������A��2 molH2(g)��1molO2(g)������������2mol H2O (l)���������÷�ӦΪ���ȷ�Ӧ��B�����ݸ�˹���ɣ���Ӧ��������ֻ��ʼ̬����̬�йأ��ͷ�Ӧ;���أ�1 mol H2O (g)�ֽ��H2(g)��O2 (g)������241.8kJ������C��1mol H2O (l)���1mo1 H2O (g)������44kJ������D����������H2O (g)��H2O(l�����������ߣ�ͬһ���ʵ���̬Ҫ��Һ̬�������ߡ�
���㣺�����˹���ɺ��Ȼ�ѧ����ʽ����ؼ��㡣
���������A��2 molH2(g)��1molO2(g)������������2mol H2O (l)���������÷�ӦΪ���ȷ�Ӧ��B�����ݸ�˹���ɣ���Ӧ��������ֻ��ʼ̬����̬�йأ��ͷ�Ӧ;���أ�1 mol H2O (g)�ֽ��H2(g)��O2 (g)������241.8kJ������C��1mol H2O (l)���1mo1 H2O (g)������44kJ������D����������H2O (g)��H2O(l�����������ߣ�ͬһ���ʵ���̬Ҫ��Һ̬�������ߡ�
���㣺�����˹���ɺ��Ȼ�ѧ����ʽ����ؼ��㡣

��ϰ��ϵ�д�
�����Ŀ
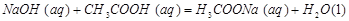
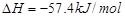
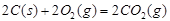
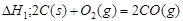
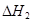 ���H1>��H2
���H1>��H2