��Ŀ����
�������е������Һ����Na2CO3 ��NaHCO3  ��CH3COONH4
��CH3COONH4
��NH4HCO3
��1���������ֵ������Һ�У������������������ռ���Һ��Ӧ���ǣ���д��ţ�
��2����֪���� �������£����ʵ���Ũ����ͬ�Ģ١��ڡ�����ҺpH��С˳��Ϊ������ţ� > >
�������£����ʵ���Ũ����ͬ�Ģ١��ڡ�����ҺpH��С˳��Ϊ������ţ� > >
��3��д����������NaOH��Һ��ϼ��ȷ�Ӧ�����ӷ���ʽ
��4����֪������CH3COONH4��Һ�����ԣ�������һ��ʵ�Ʋ����Һ��pH 7����>��=��<��������
 ��CH3COONH4
��CH3COONH4��NH4HCO3
��1���������ֵ������Һ�У������������������ռ���Һ��Ӧ���ǣ���д��ţ�
��2����֪����
 �������£����ʵ���Ũ����ͬ�Ģ١��ڡ�����ҺpH��С˳��Ϊ������ţ� > >
�������£����ʵ���Ũ����ͬ�Ģ١��ڡ�����ҺpH��С˳��Ϊ������ţ� > > ��3��д����������NaOH��Һ��ϼ��ȷ�Ӧ�����ӷ���ʽ
��4����֪������CH3COONH4��Һ�����ԣ�������һ��ʵ�Ʋ����Һ��pH 7����>��=��<��������
��1���ڢܢݣ�1�֣�
��2����>��>�ڣ�1�֣�
��3��NH4++HCO3-+2OH-
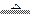 NH3��+CO32-+2H2O
NH3��+CO32-+2H2O��4��>�����ɣ���ΪCH3COONH4��Һ�����ԣ�˵��CH3COO-��NH4+ˮ��̶���ͬ����H2CO3��������CH3COOH��HCO3-��ˮ��̶ȴ���CH3COO-������NH4HCO3��ҺpH>7��3�֣�
���⿼�����ʡ�����ˮ�⼰���ӷ�Ӧ����ʽ��д����1��HCO3 �C�Ȳ����Ṳ�棬Ҳ�����棻NH4+�����棬CH3COONH4Ϊ���������Σ��ʼ��������ᷴӦ���������ռӦ�ĵ�����Тڢܢݣ���2���١��ڡ��۶�����ˮ����Σ���ҺpH�Ĵ�С��ȡ��������Һ�������ӵ�ˮ��̶ȣ�ˮ��̶�Խ����ҺpHԽ����pH����>��>�ڣ���3��NH4HCO3������NaOH��Һ��ϼ��Ȳ���������NH4++HCO3-+2OH-
 NH3��+CO32-+2H2O����4��CH3COONH4�����������Σ���Һ�����ԣ�˵��CH3COO-��NH4+ˮ��̶���ͬ����H2CO3��������CH3COOH��HCO3-��ˮ��̶ȴ���CH3COO-������NH4HCO3��ҺpH>7��
NH3��+CO32-+2H2O����4��CH3COONH4�����������Σ���Һ�����ԣ�˵��CH3COO-��NH4+ˮ��̶���ͬ����H2CO3��������CH3COOH��HCO3-��ˮ��̶ȴ���CH3COO-������NH4HCO3��ҺpH>7��
��ϰ��ϵ�д�
�����Ŀ
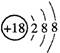
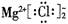
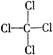

 MnO2+Zn+2H2SO4
MnO2+Zn+2H2SO4